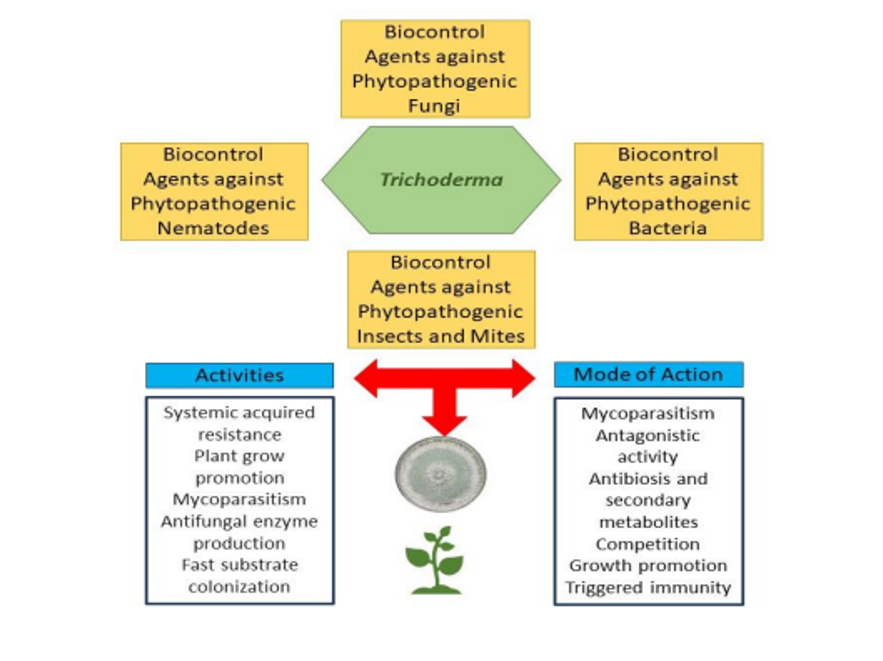
INTRODUCTION
Microbial inoculants have emerged as a promising approach for augmenting crop yield and improving the overall quality of agricultural production. Trichoderma-based products have captured the attention of researchers and agricultural producers due to their ability to enhance plant resistance to various biotic and abiotic stresses. Additionally, these products can improve the nutritional quality of crops and contribute to sustainable agriculture1.
Biofungicides, a type of biocontrol agents, have gained attention as a potential solution to reduce the negative impacts, caused by chemical or synthetic fungicides on the environment, animal life, and human health. Trichoderma has been widely adopted as a biofungicide by farmers due to its ability to limit the growth of various plant pathogens. For instance, Trichoderma has been reported to generate antibiotics and volatile compounds, and to stimulate plant resistance against pathogens. Additionally, it has been shown to compete against other microorganisms in the rhizosphere, while exhibiting mycoparasitic behavior2.
Trichoderma is a dominant active ingredient in more than half of the registered biofungicides that are produced globally, reported approximately 50%, and designed to combat soil-borne pathogens. This reflects the widespread acceptance of Trichoderma-based formulations among producers and researchers as a reliable approach for managing plant diseases caused by soil-borne pathogens. The success of these biofungicides is attributed to their effectiveness, safety and compatibility with other crop protection measures3. Trichoderma is one of the most studied antagonists as a biological control agent, as it is capable of controlling a wide range of plant pathogens including bacteria, fungi, insects and nematodes4.
This fungus is distributed in various ecosystems, where the rhizosphere represents one of the most common ecological niches as it is attracted to certain plant pathogens as prey and to the nutrients exuded by the roots of plants. It can be isolated from all types of agricultural and horticultural fields in different climatic zones due to the diversity of the genus2,3,5.
In contrast to chemical pesticides, the use of biocontrol agents have a number of advantages, for instance: i) it does not lead to the development of resistance in pathogens; ii) it avoids environmental contamination; iii) it inhibits the proliferation of secondary pests; iv) it is suitable for organic farming practices, and; v) it adheres to regulatory limits regarding maximum chemical residue levels on fruits and vegetables6.
In view of the role of this fungus in sustainable agriculture, this mini review aims to explore the beneficial aspects of various Trichoderma species, highlighting their diverse mechanisms of action.
MATERIALS AND METHODS
For the realization of this narrative literature review, databases of national and international journals indexed in Scielo, Latindex 2.0, Scopus, Scimago, and Web of Science were reviewed. Google Scholar and the digital library of the National Council for Science and Technology's Scientific Information Center (CICCO) were utilized. For the searches, keywords such as Trichoderma, biocontrol, action mechanisms, mycoparasitism, antibiosis, endoparasitism, defense response induction, biopesticide, bio fungicide, among others, were used.
Subsequently, an Excel spreadsheet was created to organize the data from the articles obtained from various databases. Duplicates were removed, and the articles were thoroughly read. The selection of articles was carried out based on the objectives set forth in this review, scientific articles related to the action mechanisms of Trichoderma as a biocontroller and its potential applications in agricultural fields were considered. Works focused on Trichoderma as a plant growth promoter were not considered.
The search period ranged from December 2022 to May 2023, and primarily articles from the last 5 years were considered. However, those regarded as worldwide references on the topic and published over 5 years ago were included in this paper. Basic references on the topic outside the specified time period were included.
To obtain information on Trichoderma-based products authorized in Paraguay, the website of the regulatory authority was consulted - Servicio Nacional de Calidad y Sanidad Vegetal y de Semillas (SENAVE).
RESULTS
In Google Scholar, using the keywords, a total of 94,500 related documents appeared on the selected topic. Refining the search to the last 5 years reduced the number of documents to 17,400. In the case of the search conducted in Scopus, 327 documents were detected, including 248 articles, 52 literature reviews, 21 book chapters, and 4 conference abstracts. Refining the search to the last 5 years reduced the number of documents to 109. Regarding the search performed in Web of Science, a total of 285 scientific papers were found, including 229 scientific articles, 55 review articles, 2 conference abstracts, 1 book chapter, and 1 rapid communication article. Restricting the search to the last 5 years resulted in 114 scientific articles. After removing duplicates and verifying that the articles were relevant to the objectives established for this review, a total of 81 materials were selected for use in this paper.
DISCUSSION
4.1 The Genus Trichoderma
Persoon published the initial account of the Trichoderma genus in 17947. As of 2022, 349 Trichoderma species had been identified and DNA sequences are available and in public databases like https://trichokey.com or and http://mmit.china-cctc.org 8.
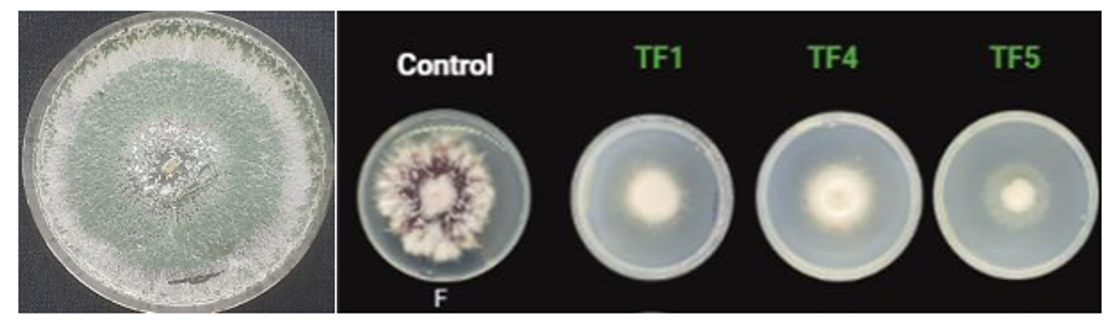
Imagen 1 (a) Pure colony of Trichoderma asperelloides (b) Trichoderma asperelloides as a biocontroller of Fusarium oxysporum (own source)
TrichodermaFigura 1 , owing to its remarkable adaptability, can be propagated on diverse solid and liquid growth media. The morphological characteristics of the colonies, such as the concentric rings and mycelial pigmentation, are subject to variation depending on the species and culture medium. The rear of Trichoderma colonies typically displays hues of colorlessness, beige, yellow, amber, or greenish yellow depending on the growth conditions and species. Thus, these organisms exhibit considerable diversity in their morphological attributes9.
These fungi reproduce asexually through spores or conidia, which are globular structures, green in color and measure approximately 3-5 µm. their resistance structures are called chlamydospores and are usually thick, with a soft green color and intercalary position. Conidiophores have perpendicular or lateral branching in several groups, are green, and measure approximately 62-69 x 3-4.7 µm. The phialides, often in pairs, are long and thin, with up to four terminal verticils of phialides9,10.
Trichoderma produces abundant conidia to maintain long periods of vegetative growth. However, the transition from mycelium to conidium is determined by a combination of factors capable of triggering this change. Nutritional environment, pH, metabolite production, light, and even the fungus' own metabolism, are some of the factors responsible for the way conidiation occurs. This process is critical for the survival of the fungus, yet it has been shown that the conidial response varies widely, depending on the metabolic adaptation of each species to the environment11.
Comparative genetic studies have demonstrated that Trichoderma has undergone genome remodeling to enhance its ability to rapidly colonize and successfully compete in new habitats. As such, it may be misleading to associate specific biological activities, such as metabolite production, with species that have been described under outdated names within the current taxonomic classification of Trichoderma12.
4.2 Mechanisms of action and biological control agent
It is important to note that the efficacy-based biocontrol agents can be improved through the selection of competent strains and optimal formulations of organisms adapted to various agroecosystems. However, it is crucial to enhance the performance levels of this antagonist and acknowledge that the outcomes of biological control rely on the interaction between the pathogen and Trichoderma. Additionally, it should be considered that Trichoderma is most effective in controlling pathogens preventively6.
According to the comparative analysis of the genomes of three Trichoderma species (T. virens, T. reesei, and T. atroviride) commonly utilized as biocontrol agents in agriculture, it has been revealed that the original lifestyle of the fungal genus was mycoparasitism4. The fungi produce various metabolites such as antibiotics, mycotoxins, and phytotoxins, which aid in its antagonistic effects. Additionally, the fungus releases enzymes such as glucanases, chitobioses, and chitinases, as well as antibiotics like viridin, gliotoxin, or peptaibols 13,14) Tabla 1
Table 1 Mechanism of action of Trichoderma as a biocontrol organism.
| Mechanism of action | Description | Pathogen specificity | References |
|---|---|---|---|
| Mycoparasitism | The hyperparasite is dependent on the host fungus and acquires nutrients through haustoria without inducing host cell death. These attacks are initiated by the Trichoderma fungi through the penetration of the cell wall of the pathogen, followed by the subsequent degradation of its cell components. Trichoderma have conventionally been regarded as necrotrophic mycoparasites, and research has mainly concentrated on the degradation of the host's cell wall. Nonetheless, a mode of action analogous to "hemibiotrophy" has been proposed, and evidence suggests that the fungal cell wall of the host is not extensively harmed during interactions with Trichoderma. Conversely, digestion and mobilization of the cellular contents appear to be critical. | Pathogen specific interaction | 14-18. |
| Antibiosis | Production of low-molecular-weight volatile or non-volatile antibiotics or diffusible compounds. These substances that inhibit or reduce the growth and/or proliferation of phytopathogens. More than 90 metabolites have been reported in Trichoderma species including Trichorzianin TA, Trichorzianin TB, 6-pentyl-2H-pyran-2-one, Trichodermin, Cyclonerodiol, Pachybasin and others. | Broad | 2,14,16,17,19-21.25 |
| Competition | Trichoderma compete with phytopathogens for space and essential nutrients. It is capable of colonizing the root rhizosphere and effectively competing with other microorganisms for nutrients secreted by the soil. In addition, it has the ability to promote plant growth. Trichoderma spp. releases siderophores that sequester Fe3+, making them inaccessible to pathogens. | Broad | 14,16,22-24. |
| Induction of plant defenses and endophytism | Activation of host defense mechanisms against diseases and other stresses. Trichoderma can interact with plants and elicit a defense response against pathogens or disease. The use of Trichoderma results in the production and accumulation of enzymes, secondary metabolites, and signaling molecules, such as salicylic acid (SA), ethylene (ET), and jasmonic acid (JA), leading to enzymatic and morphological changes within the host plant. Ultimately, this results in the induction of induced systemic resistance in the plant. The interaction between Trichoderma and the plant is dependent on various factors, including the strain, its concentration, the plant material, developmental stage, and the timing of interaction. Trichoderma contains genes that are expressed in plants to help them manage diseases and impart resistance to environmental stressors. Commonly used marker genes in this defense include PDF1.2 (Plant defensin 1.2), Thi2.1 (Thionin), or Chib (Chitinase B). The salicylic acid-mediated systemic acquired resistance (SAR) results in the expression of pathogenesis-related genes (PR). | Specific to broad | 14-17,26,27-29. |
Trichoderma employs various intricate direct and indirect mechanisms of biocontrol to combat biotic stresses posed by a broad range of pathogenic microorganisms, including fungi, bacteria, insects, and nematodes, as well as abiotic stresses resulting from unfavorable environmental conditions30. Not all Trichoderma species possess the capacity to modulate plant growth and physiology due to the wide range of symbiotic interactions between microorganisms and plants. Additionally, the response of the antagonist in terms of the production of secondary metabolites may either promote or inhibit plant growth, thus contributing to the complexity of this relationship31.
4.3 Potential biocontrol of Trichoderma in agriculture
Trichoderma has become a key ally in the integrated management of pests in crops, thanks to its diverse mechanisms for controlling without negatively impacting the environment or generating resistance in pests32.
Trichoderma has been reported as an effective agent for controlling phytopathogenic fungi for several years6; Its biocontrol effect has been mentioned against Rhizoctonia solani33; Fusarium graminearum34,35; Pythium36-38; Fusarium oxysporum4,39,40; Botrytis cinerea41-43; Sclerotium and Macrophomina44,45; Colletotrichum46-48; Sclerotinia49-51 and others.
Regarding its effect in the control of phytopathogenic bacteria, Trichoderma has been mentioned as effective for Ralstonia solanacearum52,53; Xanthomonas54,55, however, the literature referring to the control of bacteria with Trichoderma is scarce in comparison with the applications for the control of phytopathogenic fungi.
Trichoderma has been mentioned as a biocontrol agent for species of nematodes of the genus Meloidogyne including Meloidogyne incognita56-58 and others like Heterodera59.
Regarding the use of Trichoderma as an entomopathogen, its efficacy against Leucinodes orbonalis60, Regarding the use of Trichoderma as an entomopathogen, its efficacy against Leucinodes orbonalis61, Spodoptera littoralis and Macrosiphum euphorbiae62. Other authors have mentioned in reviews on the subject the control of mites, hemiptera, coleoptera, diptera, orthoptera and lepidoptera, among others4.
Interaction of Trichoderma with other beneficial organisms
Trichoderma is a well-known and extensively studied genus of fungi that plays a significant role in biological control strategies for managing plant diseases. Its interactions with other biocontrol organisms are of particular interest due to their potential synergistic effects on enhancing plant health and protection30,32,63.
This biocontroller, exhibits various modes of interaction with other biocontrol agents, such as beneficial bacteria and mycorrhizal fungi. These interactions can lead to complementary or cooperative activities that bolster the overall effectiveness of integrated pest management strategies4,64,65.
Some key aspects of Trichoderma's interactions with other biocontrol organisms include:
Antagonistic Interactions: Trichoderma is often recognized for its mycoparasitic capabilities, meaning it can attack and inhibit the growth of pathogenic fungi through mechanisms such as competition for resources, secretion of antifungal compounds, and direct parasitism. When combined with other biocontrol agents that possess different modes of action, the collective antagonistic effect can target a broader range of pathogens and contribute to more robust disease suppression7,30,32,63,66.
Synergistic Effects: Certain strains of Trichoderma have been found to enhance the performance of other biocontrol organisms. For example, when used in conjunction with beneficial bacteria, Trichoderma can promote the colonization of these bacteria on plant surfaces, thereby increasing the potential for disease prevention. This synergy can lead to improved establishment and persistence of biocontrol populations63,64.
Plant Growth Promotion: Trichoderma's ability to enhance plant growth and nutrient uptake can also complement the activities of mycorrhizal fungi. Mycorrhizal fungi form symbiotic relationships with plant roots, aiding in nutrient acquisition. Trichoderma can improve root system development, making plants more receptive to mycorrhizal colonization and leading to greater overall plant health67,68.
Induced Systemic Resistance (ISR): Trichoderma's interactions with other biocontrol agents can trigger systemic defense responses in plants, a phenomenon known as induced systemic resistance. This mechanism enhances the plant's innate ability to ward off pathogens, making it more resilient to disease attacks. When integrated with other biocontrol agents, this systemic defense response can create a multi-layered defense strategy for plants30,66,69,70.
Environmental Adaptation: Different biocontrol organisms, including Trichoderma strains, possess varying environmental tolerances and preferences. In some cases, combining strains adapted to different conditions can result in broader and more reliable disease management across diverse environmental settings71-75.
In Paraguay, a variety of products based on Trichoderma are commercially available and described in Table 2, which lists products legally registered by the Servicio Nacional de Calidad y Sanidad Vegetal (SENAVE). These products are recommended for different crops and pathogens, containing various species of Trichoderma as active ingredients. They are primarily marketed for the control of phytopathogenic fungi and nematodes76
| Product and Ref. | Trichoderma specie | Formulation | Use class | Toxicology | Manufacturer | Origen | Pathogens | Crops | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ECOTRICH | T. harzianum | WP Wettable Powder | Biological fungicide | IV | Ballagro Agro Tecnología Ltda | Brazil | Sclerotinia sclerotiorum, Macrophomina phaseolina | Soybean, alfalfa | 76,77. |
| TRICHODERMIL WP 1306 | T. harzianum | WP Wettable Powder | Biological fungicide and nematicide | III | Koppert Do Brasil Holding Ltd. | Brazil | Rhizoctonia solani, Fusarium solani, Sclerotinia sclerotioum, Thielaviopsis paradoxa, Meloidogyne sp. and Pratylenchus sp. | Extensive and intensive crops | 76,78. |
| STIMUCONTROL | T. harzianum | CS Concentrated Suspension | Biological fungicide | III | Simbiose Industria E Comercio De Fertilizantes E Insumos Microbiológicos Ltda | Brazil | Rhizoctonia solani, Sclerotinia clerotiorum | Extensive and intensive crops | 76,79. |
| RIZODERMA | T. harzianum | AL Liquid | Fungicide | IV | Rizobacter Argentina S.A. | Argentina | Cercosporakikuchii Phomopsis Fusarium spp. Fusarium spp. Alternaria spp. Bipolaris spp. Fusarium graminearum Drechsleratriticirepentis Bipolarissorokiniana Tilletialaevis Ustilagotritici | Soybean, rice, wheat | 76,80. |
| LALSTOP QUALITY WG | T. asperellun | WP Wettable Powder | Microbiological fungicide | III | LallemandSoluçõesAgrobiológicas Ltda. | Brazil | Botrytis cinérea, Didymellabryoniae, Pythium, Rhizoctonia, Phytophthora and Fusarium, Verticillium, Macrophominaphaseolina | Large variety of fruit and vegetable crops such as tomato, pepper, cucumber, lettuce, herbs and ornamentals, cucurbit crops such as cucumber and melons, herb and ornamental major fruit, strawberry and sweet potato. | 76,81. |
| BIO-R1 | T. asperellun | EC EmulsifiableConc-entrate | Microbiological fungicide | IV | Vittia Fertilizantes E Biológicos S.A. | Brazil | No data | No data | 76. |
| HULKGREEN | T. harzianum | CS Concentrated Suspension | Biological fungicide | IV | Agro Advance Technology S.A. | Argentina | Fusarium sp., Sclerotinia sp. | Extensive and intensive crops | 76,82. |
| RIZODERMA MAX | T. harzianum | AL Liquid | Therapic fungicide for seed treatment | IV | Rizobacter Argentina S.A. | Argentina | Cercosporakikuchii Phomopsis Fusarium spp. Fusarium spp. Alternaria spp. Bipolaris spp. Fusarium graminearum Drechsleratriticirepentis Bipolarissorokiniana Tilletia laevis Ustilago tritici | Soybean, rice, wheat | 76,80. |
| BIO-FORCE | T. asperellun | EC EmulsifiableConc-entrate | Nematicide, microbiological fungicide | IV | Vittia Fertilizantes E Biológicos S.A. | Brazil | No data | No data | 76. |
| TRICHOMBAT | T. atroviride | WP Wettable Powder | Microbiological fungicide | IV | Innova Ltda | Brazil | No data | No data | 76. |
| AQ® TRC | T. capillare | AL Liquid | Biofungicide | Not applicable | Aquafree S.R.L | Paraguay | Phytophthora sp., Rhizoctonia sp., Sclerotium sp. Fusarium sp., Rosellinia sp., Botrytis sp., Alternaria sp., Cercosporasp., Colletotrichum sp., Peronospora sp., Oidium sp., Pyricularia sp. | Soybean, rice, corn, sorghum, rice, pastures, cotton, sugar cane, sesame, chia, yerba mate, bananas, perennial plants, horticultural and ornamental crops | 76,83. |
Table 2. Trichoderma based commercial bioformulations registered in Paraguay
CONCLUSION
The potential of Trichoderma species to foster sustainable agriculture is of paramount importance. These fungi exhibit a myriad of mechanisms that can profoundly influence soil health, plant development, and the ability to ward off diseases. Trichoderma's multifaceted role as a biocontrol agent, enhancer of nutrient assimilation, and fortifier against stress holds great promise for ushering in sustainable agricultural paradigms.
Moreover, the application of Trichoderma as a biofertilizer offers a pivotal advantage by diminishing reliance on synthetic fertilizers notorious for their environmentally detrimental impacts. This collective prowess positions Trichoderma as a compelling and environmentally conscious substitute for conventional agricultural methodologies.
Furthermore, the wealth of scientific articles available online underscores the extensive body of research dedicated to unraveling the potentialities of Trichoderma. These articles expound on the intricate ways these fungi can augment soil microbial communities, amplify plant growth, and bolster disease resistance. This robust scientific foundation not only validates Trichoderma's efficacy but also highlights the ongoing commitment to harnessing its capabilities for sustainable agricultural advancement.
The dynamic attributes of Trichoderma species underscore their potential as a cornerstone of sustainable agricultural practices. Their capacity to revolutionize disease control, amplify nutrient efficiency, and alleviate ecological strain paves a progressive path toward a greener and more sustainable future in agriculture.











 uBio
uBio