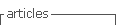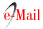INTRODUCTION
It has been more than 20 years since the first study on posterior reversible encephalopathy syndrome (PRES) was published by Hinchey et al.(1); nowadays, PRES is known as a potentially reversible clinical-radiological disorder, which usually progresses with classical acute neurological symptoms like visual impairment, acute headache, altered level of consciousness, seizures, and even focal neurological deficit(2). It is associated with clinical scenarios of acute changes in arterial pressure, hypertensive disorders associated with pregnancy, sepsis, kidney disease, autoimmune diseases (e.g., SLE), and drugrelated conditions (immunosuppressive agents, cytotoxic drugs), among other causes(3,4). The natural history of symptoms is reversal; however, this syndrome may not always be reversible and may develop atypically, causing permanent neurological sequels(5).
Regarding its radiological correlation, computerized tomography (CT) scans may evidence subtle hypodense areas; however, brain MRI is currently the “Gold Standard” for PRES diagnosis. A vast majority of PRES cases affect the parietal and occipital lobes; however, it has been proved that it may affect different brain areas(6).
There is no treatment for this condition, and its management focuses on solving its underlying cause such as measures to control arterial pressure, anticonvulsant therapy, discontinuation of drugs administered, and treatment of triggering factor (sepsis, eclampsia, and preeclampsia), however, certain studies support the use of nimodipine in vasospasm cases(7).
CLINICAL CASE
A 21-year-old woman, housewife, G2C2, with a history of systemic lupus erythematosus since 2016, treated with prednisolone and hydroxychloroquine with a low adherence to treatment and multiple immunosuppressant schemes since 2019 and lupus nephritis class IV-G (A/C) A 18/24 and C 4/12 (ISN/RNP) classification, go to the emergency service on January of 2021 because of progressive, acute bifrontal headache which increased with physical effort, as well as visual alterations, hypertensive crisis (210/110 mmHg) and a focal to a bilateral tonic-clonic seizure. She was admitted to the intensive care unit and administered IV phenytoin, endovenous nitrates, and oral antihypertensive medica tion. After recovery, she was transferred to the general ward with generalized edemas, normal neurological examination, simple brain CT with no evident pathologi cal findings, and a brain MRI showing no abnormalities (Figure 1). Other tests showed an increase in creatinine levels to 1.5 mg/dL, hypocomplementemia C3:56 and C4:18, 26 g proteinuria in 24-h urine, and a score of 44 determined by SLEDAI-2K, for this reason, received pulse IV methylprednisolone therapy for three days, and a 500 mg single dose of cyclophosphamide; her edemas and headache improved, her pressure levels were within target limits (120/80 mmHg), and she was discharged from hospital to receive outpatient treat- ment.
On July 2021, she was readmitted to the ICU because of the recurrence of acute, pulsatile, bifrontal headache, visual changes, seizure, and severe HBP (220/110), as well as the mono paresis of the right lower limb with 2/5 muscle strength. The brain MRI taken on showed hyperintensity of the subcortical bilateral parie- tal lobes in the FLAIR and T2 sequences representing vasogenic edema, gray matter hyperintensity in the left medial parietal lobe associated with restricted diffusion suggesting cytotoxic edema and representing a brain infarct in the anterior cerebral artery area, finding su ggestive of PRES (Figure 2). During hospitalization, a second 500 mg dose of cyclophosphamide was admi- nistered. Clinical improvement was observed, absence of headache, no visual alterations, and no seizures, along with gradual recovery of strength in her lower right limb, which correlates with a decrease in the va- sogenic edema observed in a control brain MRI took 15 days after hospitalization. Our patient presents clinical improvement for 10 days, with pressure levels reaching target limits, no seizures, and no visual alterations. Be- cause of phenytoin intolerance, the neurology depart- ment decides to change the anticonvulsant therapy for levetiracetam. Subsequently, her kidney function progressively deteriorates until reaching serum creatinine levels of 3.6 mg/dL, the persistence of hypocomple- mentemia with no response to corticoid and cyclophos- phamide treatment. Biological therapy with anti-CD20 antibodies is initiated, with 500 mg Rituximab infusion as a first dose. Another seizure episode occurs, asso- ciated with high-pressure levels. Again, the neurology unit optimizes the anticonvulsant therapy and takes a new MRI scan, in which the following is evidenced: enlargement of T2 interval and hyperintensity of the sub cortical bilateral parietal lobe, suggesting an increase in the vasogenic edema, and Gyriform hyperintensity observed in the T1 scans suggests laminar necrosis of the left ACA area. An interval resolution of the restricted diffusion of the gray matter in the left parietal lobe suggests chronic changes in the left ACA infarct (Figure 3).
Figure 2 MRI of the brain dated 02/07/2021 showed A: Flair. B: T2 C: Diffusion D: ADC map. The white matter at the parietal occipital subcortical region shows hyperintense images in T2 sequences representing vasogenic edema typical of PRESS syndrome. In addition, in the medial part of the left parietal lobe, there is a hyperintense lesion in T2 which, unlike the other lesion, is gray matter and has diffusion restriction indicating a cerebral infarction in the territory of the anterior cerebral artery, turning it into a PRES complicated.
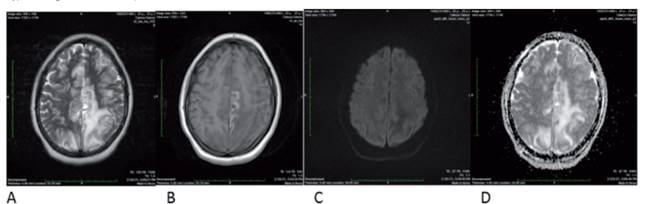
Figure 3 MRI of the brain dated 02/28/2021 showed A: T2 B: T1 C: Diffusion D: ADC map. The infarction of the territory of the anterior cerebral artery has made a normal evolution with cortical necrosis, and disappearance of the diffusion restriction, which are expected changes in a cerebral infarction, however, the vasogenic edema of the parietal lobes has increased and has extended to the right medial parietal side. No diffusion restriction indicates that it is a typical vasogenic edema of simple PRES.
Regarding her kidney function, a gradual decrease in nitrogen compounds and serum creatinine levels was observed, her last levels being 0.9 mg/dL, thus sho wing a good response towards Rituximab administration. A satisfactory evolution is observed for five days, with pressure levels controlled by the use of three oral antihypertensives, without headaches or visual alterations, and with normal nitrogen compound levels. On discharge from the hospital, she is prescribed oral corticoids and scheduled for a Rituximab infusion within 30 days.
DISCUSSION
Syndrome PRES is known as a clinical radiological disorder of reversible subcortical vasogenic brain edema in patients with classical acute neurological symptoms related to hypertensive disorders associated with pregnancy, sepsis, kidney disease, autoimmune disease, hypertension, drug administration (immuno- suppressive-cytotoxic agents), among other causes, mostly observed among women(5).
Patients with this syndrome develop an acute neuro logical condition associated with focal and/or generalized seizures, as well as global headache associated with visual disorders (photophobia, blurred vision, di plopia, scotoma, hemianopsia, amaurosis, and even cortical blindness), vomiting syndrome, emesis, confusion, and focal neurological signs such as hemiparesis and sensitive deficit(8). These symptoms tend to be clinically and radiologically reversible with a favorable prognosis. However, a few patients may experience long-term neurological complications such as focal deficits secondary to the persistence of brain vasoge- nic edema. The latter is usually associated with cases caused by a hypertensive crisis in 20% of cases(9,10), however, PRES may occur without hypertension (25%- 30%).
PRES usually occur as a single episode, which isusually reverted once the triggering noxa has been controlled. However, there is a 4%-12% risk of developing recurrence within the next 15 days to 36 months following the first episode(10,11).
In a study published in 2018, patients with SLE, lupus nephritis, and corticosteroid or cyclophosphamide use were associated with PRES development(12,13) as in the case of our patient with a history of SLE and lupus nephritis under treatment with corticosteroids and cyclophosphamide. More recently, a case series review conducted on 13 patients to describe the clinical manifestation, medical history, comorbidities, and treat- ments associated with patients with posterior reversible leukoencephalopathy evidenced that the average age of occurrence was 13 to 57 years old. The main comorbidities included HT (46%), SLE (23%) and all SLE patients presented impairment of the kidney function along with hypocomplementemia and were positive for anti-DNA antibodies(14).
Rituximab (anti-CD20 monoclonal antibody) is used for treating patients with non-Hodgkin’s lymphoma, and systemic conditions such as systemic lupus erythematosus, lupus nephritis, and sarcoidosis. Different case report publications evidenced the association between Rituximab and PRES, even within a few hours following infusion(15), usually followed by improvements after 48-72 h; in our case, the appearance of the PRES syndrome was associated with the application of rituximab, however, it is not possible to establish a direct association since the patient was receiving other treatments such as cyclophosphamide and corticosteroids(16-19).
Brain MRI is the “Gold Standard” for PRES diagnosis, and unlike CT scans, MRI allows to observation of smaller injuries such as isointense or hypointense areas in T1 or hyperintense areas in T2, as in our patient. FLAIR sequences allow for a better distinction between white matter and cerebral cortex damage. DWI imaging and ADC maps are useful for early diagnosis because they can differentiate irreversible cytotoxic edema from reversible vasogenic edema. In mild cases, it tends to develop at a cortical-subcortical level, and it is primarily localized in the bilateral parieto-occipital area, followed by frequency of occurrence by the superior posterior frontal area, temporal area, cerebellum, basal ganglia, and brainstem. A brain MRI control is usually recom- mended after 30 days, which will depend on the persistence of symptoms or their full remission(20).
The prognosis of these patients primarily depends on the early detection of PRES and on the commencement of timely control of the noxa causing this condition. In general, PRES prognosis is favorable, and mostpatients recover both at a clinical and imaging level. However, atypical/complex cases have been reported, with irreversible damage, intracranial hemorrhage, and intracranial hypertension. Overall, data estimate a mor tality rate of up to 6%(5,21-25).
In the case reported in this study, an association between autoimmune diseases such as SLE and PRES has been evidenced, correlating risk factors such as being a young woman with renal impairment and HBP, receiving chronic immunosuppressive treatment with oral corticoids, azathioprine, and cyclophosphamide, as well as the use of anti-CD20 biological therapy with Rituximab. Our patient had two hypertension crises associated with the acute neurological syndrome, which were timely reverted with antihypertensive treatment. Moreover, it was found that 10 days after a gradual improvement of symptoms and pressure levels within target limits, she had another PRES episode following the administration of biological therapy with Rituximab, which had been initiated to prevent the progression of her permanent chronic kidney disease secondary to class IV lupus nephritis refractory to systemic corticoids and cyclophosphamide. From a radiological perspective, the clinical-radiological correlation observed in our patient suggested a recurrent atypical pre- sentation of the disease. Image 2 shows an MRI with the following combination of radiological results: at the parieto-occipital level, in the subcortical area, the white matter observed with T2 sequence shows hyperintense images representing a vasogenic edema characteristic of PRES. However, in the medial part of the left parietal lobe there is a hyperintense injury in T2, although, con- trary to the findings in the other lesion, this was reported in the gray matter and had restricted diffusion, suggesting a cerebral infarction in the anterior cerebral artery area. This indicates that the disease was no longer a simple PRES and became a complex PRES because the patient suffered from irreversible ischemic damage and PRES is, by definition, reversible.
Figure 3 shows that the anterior cerebral artery infarct evolved normally with cortical necrosis and that the diffusion restriction disappeared. These changes are normally expected in cerebral infarction. However, the parietal lobes‘ vasogenic edema has increased and spread toward the right medial parietal side. There is no restricted diffusion, suggesting the presence of va- sogenic edema typical of simple PRES. These results suggest an evolving left ACA infarct with a new episode of PRES syndrome, most probably due to the Rituximab administration.
Based on the results of this case, it could be said that posterior reversible encephalopathy syndrome (PRES) may be associated with multiple factors, as it was previously stated. Moreover, a clear correlation between PRES and hypertensive crisis, the activity of autoimmune diseases such as SLE with severe renal function impairment, and the use of biological therapies such as Rituximab can be observed. Furthermore, a clinicalradiological correlation is observed following the optimization of triggering factors. Finally, we could describe a case of complex PRES associated with a left ACA infarct in a patient with lupus nephritis, an interesting case that has been scarcely described in the literature.