Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Cirugía paraguaya
versão On-line ISSN 2307-0420
Cir. parag. vol.47 no.2 Asunción ago. 2023
https://doi.org/10.18004/sopaci.2023.agosto.34
Case report
Pseudopapillary tumor of the pancreas. A case report
1Ministerio de Salud Pública y Bienestar Social, Centro Médico Nacional, Hospital Nacional, Departamento de Cirugía. Itauguá, Paraguay
The pseudopapillary tumor accounts for 1 to 2% of all exocrine neoplasms of the pancreas. It has a higher incidence in young women and grows slowly. We present the case of a 41-year-old female patient with a history of 4 weeks of pain in the epigastrium and right hypochondrium, along with nausea and vomiting, without jaundice or weight loss. She underwent laparoscopic corporocaudal pancreatectomy.
Keywords: Cystadenocarcinoma; Frantz tumor; pancreatic cyst.
El tumor pseudopapilar representa el 1 a 2% de todas las neoplasias exocrinas del páncreas. Tiene mayor incidencia en mujeres jóvenes y es de crecimiento lento. Se presenta el caso de paciente de sexo femenino, de 41 años, con antecedente de dolor de 4 semanas de evolución, a nivel de epigastrio e hipocondrio derecho, náuseas y vómitos sin ictericia ni pérdida de peso, sometida a pancreatectomía corporocaudal laparoscópica
Palabras claves: Cistoadenocarcinoma; tumor de Frantz; quiste de páncreas
INTRODUCTION
Solid pseudopapillary tumor of the pancreas is one of the less common neoplasms among the exocrine tumors of this gland. It was first described by Frantz in 1959 as a papillary tumor of the pancreas. In 1996, the WHO renamed it as SPT within the international histological classification of tumors1.
It comprises only 0.2 to 2.7% of all pancreatic tumors. Its etiology is uncertain, and it occurs in young women between 18 and 40 years of age. The clinical manifestation is a slowly growing abdominal mass without abdominal pain. Its preferred location is the tail of the pancreas, followed by the body2. They have low malignant potential; however, some cases can be locally aggressive and invasive, with metastases to the liver, lungs, and skin3.
The imaging characteristics of Frantz's tumor include solid and cystic components, intratumoral hemorrhage, fibrous capsule with degenerative changes, and occasionally calcifications. These features make it possible to differentiate this tumor from other pancreatic tumors4. The classical histological pattern consists of papillary tumor cells composed of a fibrovascular stalk surrounded by several layers of epithelial cells that contain small blood vessels, thus forming pseudopapillae5.
CASE PRESENTATION
A 41-year-old female patient presented with a 4-week history of pain in the epigastrium and right hypochondrium, described as heaviness of moderate intensity, radiating to the right dorsal region. She also reported general fatigue, nausea, and vomiting on several occasions with liquid content; no weight loss, fever, or jaundice was reported. She mentioned similar episodes of pain over the past 14 years, which were relieved by common analgesics, and she had undergone conventional cholecystectomy 7 years ago. Physical examination revealed a globular, asymmetric abdomen due to Kocher subcostal and Pfannenstiel scars; the abdomen was soft, depressible, mildly painful on deep palpation in the periumbilical region, where an approximately 6 x 10 cm poorly defined solid elastic tumor was palpable, with its edges extending under the costal margin, showing pre-tumoral resonance, no movement with respiration, and no changes with Valsalva maneuvers.
Laboratory analyses showed: hemoglobin 13.3 g/dL, hematocrit 39%, glucose 262 mg/dL, Hb1Ac 9.3%, normal liver function tests, alpha-fetoprotein 1.26 ng/mL, CEA 1.8 ng/mL, CA 15-3 7 U/mL, CA 125 13 U/mL.
The ultrasound report indicated: a hypoechoic solid nodular tumor with regular borders, measuring 72 x 76 mm, located in the projection of the pancreatic tail with extension towards the splenorenal space; peripheral and central vascular signals were observed on color Doppler. No dilatation of the common bile duct was noted.
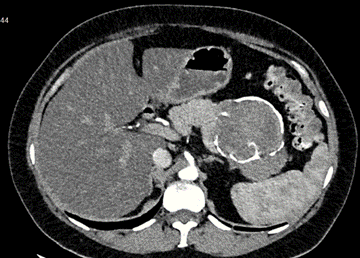
Contrast-enhanced CT revealed a lobulated rounded mass in the projection of the body and tail of the pancreas, measuring approximately 11 x 8 x 7 cm. The mass showed predominantly peripheral thick calcifications, heterogeneity, cystic appearance, and areas of higher density; internal papillae were indiscernible. No contrast enhancement was observed in arterial, portal, or delayed phases. No perilesional lymphadenopathies were noted (Figure 1). The report suggested complementing with MRI to better determine the intracystic characteristics of the tumor.
MRI findings expressed an oval-shaped polylobulated mass at the level of the pancreatic tail, measuring 125 mm transverse diameter, 84 mm anteroposterior diameter, and 84 mm in height. The mass exhibited irregular peripheral enhancement with irregular contrast uptake in intralesional areas, possibly corresponding to internal pseudopapillae (Figure 2).
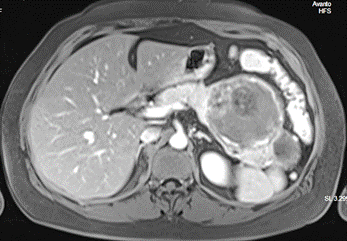
Figure 2. Abdominal MRI image of a rounded mass with lobulated contours, measuring approximately 11 x 8 x 7 cm.
The surgical procedure performed was a laparoscopic corporocaudal pancreatectomy with splenectomy: a tumor measuring 12 x 8 x 8 cm was found in the body and tail of the pancreas, with no macroscopic lymph node involvement; it was firmly adhered to the splenic vein, which appeared thrombosed. The surgical time was 4 hours (Figure 3).
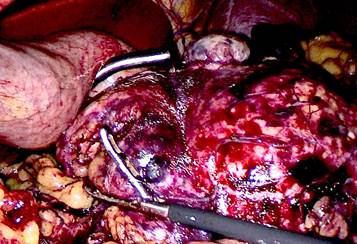
Figure 3. Tumor measuring 12 x 8 x 8 cm located in the body and tail of the pancreas, without macroscopic lymph nodes.
The pathology report indicated: epithelial tumor with medium-sized polygonal cells, eosinophilic cytoplasm, and mildly atypical central nuclei; they were arranged in solid or cystic areas with hemorrhage or pseudopapillary patterns. The cells were strongly positive for vimentin and CD56, with additional areas staining with B-catenin and partially with CD10. No vascular invasion of the splenic vein was observed.
The patient had a favorable postoperative course in the initial days. On the fifth day, she developed a low-output pancreatic fistula that was managed with nutrition and antibiotic therapy. Spontaneous closure occurred on the twelfth postoperative day, which was also the date of her discharge.
DISCUSSION
The pseudopapillary tumor of the pancreas is characterized by slow growth. In this patient's case, the tumor was not noticed in preoperative studies conducted prior to a conventional cholecystectomy 7 years ago1). The tumor has low malignant potential but can metastasize in 15% of cases, especially in patients with poor prognostic criteria such as male gender and advanced age6. Size is not indicative of poor prognosis; in this patient, despite the 12 cm size, curative surgery was proposed.
The performed surgery was a corporocaudal pancreatectomy. The laparoscopic approach was used due to the patient's age and absence of poor prognostic signs3. Macroscopically, there appeared to be involvement of the splenic vein, leading to a combined splenectomy to reduce the 15% risk of metastasis mentioned earlier, although this was ruled out by pathological anatomy2,6.
The laparoscopic approach is safe in these patients, providing satisfactory oncological margins, improving postoperative recovery by reducing complications associated with large abdominal incisions, and maintaining a reasonable surgical time.
REFERENCES
1. Begoña-Álvarez C. Tumor de Frantz o neoplasia sólida seudopapilar de páncreas. gastroenterologia y hepatologia, 2015. 38 (7): 468-470. [ Links ]
2. Jiménez-Fuertes M, Ramírez-García J, Ruiz-Tovar J, Díaz G, Durán-Poveda M.Neoplasia sólida pseudopapilar de páncreas. Cirugía Española. 2016. 94(2): 31-33. [ Links ]
3. Barreda-Bolaños F. Tumor sólido pseudopapilar de páncreas: Tumor de Frantz. Horizonte Medico, 2018. 18 (2): 80-85 [ Links ]
4. Tafur A, Suarez D. Tumor de Frantz: el tumor de las mujeres jovenes. correlacion radiologico-patologica de dos casos en tomografia revision de la literatura. Revista medica, 2017; 25 (1): 70-77. [ Links ]
5. Llatas J, Frisancho A. Tumor de Frantz: neoplasia sólida pseudopapilar de páncreas. Revista gastroenterologia del peru, 2011; 31(1): 56-60. [ Links ]
6. Wang P, Wei J, Wu J, Xu W, Chen Q, Gao W, Miao Y. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas: A single institution experience with 97 cases. Pancreatology 2018; 18(4): 415-419. [ Links ]
6Conflicts of interest: The authors declare no conflicts of interest and adhere to ethical conduct and good publication practices. No external financial support was received.
7Authors' contributions: MAAW contributed to data collection and analysis for the work; literature search, writing of the work, critical review and final approval; and agrees to be accountable for all aspects of the work to ensure that issues related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RA contributed to conception or design of the work, data collection and analysis for the work; literature search, writing of the work, critical review for important intellectual content, and final approval.
Received: November 18, 2022; Accepted: April 28, 2023










 texto em
texto em