Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cirugía paraguaya
versión On-line ISSN 2307-0420
Cir. parag. vol.47 no.1 Asunción abr. 2023
https://doi.org/10.18004/sopaci.2023.abril.7
Original article
Knowledge on placement criteria and management of pleural drainage tube in different conditions by residents of different medical specialties, Hospital de Clínicas. Year 2021
1Universidad Nacional de Asunción, Facultad de Ciencias Médicas, Hospital de Clínicas, Segunda Cátedra de Clínica Quirúrgica. San Lorenzo, Paraguay
Introduction:
With the outbreak of the SARS-CoV-2 pandemic, an exponential increase of respiratory conditions was seen and associated with it, the arrival of several pleural conditions as a complication of respiratory conditions like the placement of pleural drainage.
Objective:
to assess the know-how regarding criteria for placing and managing pleural drainage in residents from different medical specialties at Hospital de Clínicas, Paraguay.
Materials and methods:
Non-probability convenience sampling method was used including a survey of all residents from Hospital de Clínicas, Paraguay.
Results:
A total of 185 residents responded to the survey. It showed that 23.2% (43) would place pleural drainage to treat malignant pleural effusion, 86% to treat tension pneumothorax, and only 1.6% (3) to treat a pneumothorax with clinical repercussions although the latter is the most solid indication for placing a chest tube drain system (CDS).
Conclusion:
there is still controversy on the indications for placing a pleural drainage and prescribing treatment.
Keywords: Chest; Pleural cavity; Pleura; Pleural effusion; Thoracostomy.
Introducción:
Con la pandemia del Sars-CoV2 se observó un aumento exponencial en las patologías respiratorias y, con ello, pleurales tales como complicaciones de cuadros respiratorios como la colocación de drenajes pleurales.
Objetivo:
Evaluar el conocimiento existente sobre criterios de colocación y manejo del drenaje pleural en los residentes de diferentes especialidades del Hospital de Clínicas.
Materiales y métodos:
El muestreo fue no probabilístico de conveniencia. Se encuestó a todos los residentes del Hospital de Clínicas.
Resultados:
Se encuestó a un total de 185 médicos residentes, de los cuales el 23.2% (43) colocaría un drenaje pleural para tratar derrames pleurales malignos, sólo el 86% para tratar neumotórax a tensión por tan solo el 1,6% (3) para trarar neumotórax con repercusión clínica, cuando esta es la indicación más contundente para la colocación de un TDP.
Conclusión:
existe disparidad de criterios en cuanto a las indicaciones para la inserción y cuidados de los drenajes pleurales.
Palabras clave: tórax; cavidad pleural; pleura; derrame pleural; toracostomía.
INTRODUCTION
The prevalence of pleural diseases is somewhere between 4% and 10%1 compared to all respiratory conditions, and have a local or systemic etiology. Based on their etiology, clinical-surgical management is required, and many times with different approaches.
After the outbreak of the SARS-CoV-2 pandemic in 2019 until present time, an exponential increase of respiratory conditions and, consequently, pleural disease has occurred as a complication of respiratory conditions, mainly after the third week of the disease when bronchiectasis, pleural thickening, and pleural effusion2 were predominant. It became evident how little did we know on how to manage the water-seal chest tube drainage system (CDS).
It is the general or the thoracic surgeon the physician responsible for placing chest tube drainage systems. However, all physicians should be able to recognize and understand the indications that a patient should meet to discuss the possibility of proceeding with a CDS. Also, on a routine daily basis, the patient’s treating physicians should remain vigilant of all pleural outputs and their characteristics, thus assessing all possible complications that may occur immediately after placing the CDS.
Chest tube drainage systems were already discussed in Hippocratic texts for the first time back in the 5th century B.C. Water-seal chest tube drainage system was first described by Playfair back in 1875. However, its systematic use to treat empyema was first implemented by the German physician Dr. Gotthard Bülau (1835-1900) back in 1876. The repercussion and popularity gained by this method was so significant that, in modern medicine, the name of this doctor is closely associated to water-seal chest tube drainage systems. This unidirectional system expanded the lung gradually, which spared many thoracotomies and thoracoplasties at the time. In 1910, Robinson added suction to this system using vacuum pumps3.
Nearly 186 years have passed since this chest tube drainage system was first used systematically. Still, there is much controversy surrounding the management of these systems and when the best time to place them is. Therefore, surgeons, intensivists, and emergency doctors all need to be aware that these questions are still looming. Doctors at the ED play a crucial role too since placing a CDS often means the difference between life and death for the patient in just a matter of seconds.
The most common indications for pleural space drainage are situations associated with the presence of fluid deposits in that cavity like air (pneumothorax), blood (hemothorax), lymph (chylothorax), pleural fluid (pleural effusion), pus (empyema), and a combination of the former, among other. These fluids occupy space inside a cavity that cannot dilate itself, resulting in the lung passive collapse with the corresponding changes to the patient’s cardiovascular and respiratory physiology. However, these accumulations of fluid can be managed and properly drained using CDSs. The most common situations we often find are thoracic organ traumas, spontaneous pneumothoraxes, and pleural effusions of any etiology. Similarly, drainage systems of the pleural cavity are used after thoracic surgeries to prevent fluid collection from happening4.
The overall rate of complications after placing CDSs is somewhere between 3.4% and 36%5,6,7. Complications are due to infections or associated with position or insertion. The insertion ones are immediate and associated with placing the chest tube. The positional ones occur in the short-term when the tube has been placed incorrectly inside or outside the pleural cavity, thus hindering its proper functioning. Complications due infections have a late onset and are associated with insertion itself or with the pleural cavity site. Clinical and radiological follow-up are of paramount important to monitor disease progression after placing of a water-seal chest tube drainage system. Also, it is important to know how to interpret the information from the imaging modalities performed.
A retrospective study conducted revealed the rate of complications associated with the placement of CDSs performed by residents from different areas. Compared to non-surgical residents, surgical residents had significantly fewer chances of having a complication. Also, a tendency was seen towards emergency medicine residents having more chest tube insertion complications (40%) compared to 7.1%, 25%, and 12.5% of general surgery residents, surgical residents, and GPs and internists during their rotations at the ICU setting. Emergency medicine residents more than doubled the chances of having complications compared to all remaining residents. Also, no differences were seen between emergency medicine residents accredited for 5 years and those with 1-year accreditations7. This tells us that complications are present in a high percentage of cases, which means that it’s essential to know and investigate them. Our main objective when this study was conducted was to assess the know-how surrounding the criteria regarding the placement and management of CDSs in residents from different specialties at Hospital de Clínicas in Asunción, Paraguay.
MATERIALS AND METHODS
The study design was observational, descriptive, and cross-sectional. The sample used was non-probability convenience sampling. It consisted of asking all residents with different years of training from all different medical specialties at Hospital de Clínicas, San Lorenzo, Paraguay.
All those residents who were not stationed at Hospital de Clínicas, San Lorenzo, Paraguay when data curation process started or those who refused to participate in the survey met the study exclusion criteria.
Regarding the size of the sample, all residents from 1st, 2nd, 3rd, and 4th year from the different medical specialties were included in the survey.
The following qualitative variables were considered: sex, medical specialty, year of residency, approach used to treat different pleural diseases, and right decisions made regarding future complications associated with CDS placement. Information was collected through a questionnaire that included multiple choice questions.
Statistical analysis and data management collected were arranged and processed on a Microsoft Office Excel 2016 spreadsheet.
RESULTS
A total of 185 residents were surveyed, 25.4% (47), 15.2% (28), and 59.4% (110) of whom were on the 1st, 2nd, and 3rd year of their medical residencies, respectively.
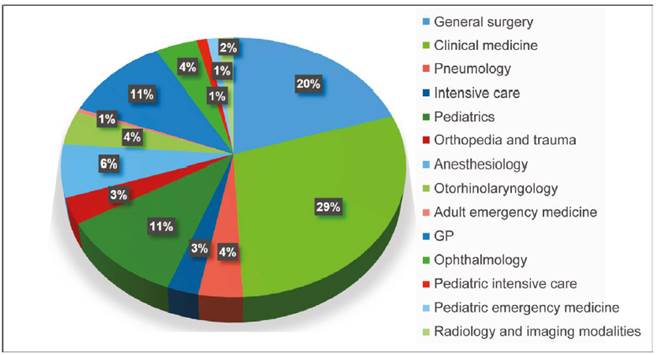
The residents’ medical specialties included general surgery, 20% (37); clinical medicine, 29% (54); pneumology, 3.8% (7); adult intensive care, 2.7% (5); pediatrics, 11% (20); orthopedia and trauma, 3.2% (5); anesthesiology, 6.5% (12); otorhinolaryngology, 4.4% (8); emergency medicine, 0.5% (1); GP, 10.9% (20); ophthalmology, 4.3% (8); pediatric intensive care, 1% (2); pediatric emergency medicine, 1% (2), and radiology and imaging modalities, 1.7% (3). (Chart 1).
Out of all the respondents, 51.9% (96) had seen themselves in a position to having to place a CDS at one time or another. Regarding the decision-making process surrounding pleural disease, 94% (174) said they would place a CDS in a patient with empyema while 86.5% (160) would do so to treat a hemothorax (160). They would all place a CDS for the treatment of tension pneumothorax; 45.9% (85) would do so to treat a grade II pneumothorax, 53.5% (99) to treat chylothorax while 49.7% (92) of respondents would place a CDS to treat pleural effusion with characteristics of exudate, 23.2% (43) to treat a malignant pleural effusion, 9.7% (18) to treat a paraneoplastic effusion, 7.5% (14) for the treatment of pleural effusion with characteristics of transudate, and 1.6% (3) to treat pneumothorax with clinical repercussions, parapneumonic effusion, and partitioned pleural effusion. None of the respondents would place a CDS to treat a minimum pneumothorax (Chart 2).
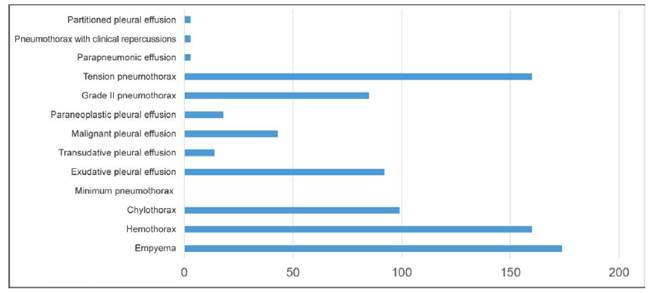
Chart 2. Decision-making process regarding the management of different pleural diseases according to residents from Hospital de Clínicas, San Lorenzo, Paraguay, 2021. N = 185
Regarding the management of water-seal chest tube drainage systems (CDS), 48% (89) said that the tube should be placed 2 cm below water level while 38.5% (71) said it should be placed 5 cm below water level, 11.5% (21) that it should be totally sealed underneath the water, and 2% (4) said that the water container should contain, at least, 500 cc of a physiological saline solution at 0.9%.
Regarding when the bottom of the water container should be removed, 5.6% (110), 7.7% (14), and 5.8% (11) of respondents said it should be changed every 24, 12, and 6 hours, respectively. Also, 21% (39) said it would depended on the amount of pleural output produced while another 5.8% (11) of respondents said it should be removed but couldn’t remember when.
Regarding count of the pleural output obtained with the CDS, 96.2% (178) said it was necessary to monitor the output while 3.8% (7) said otherwise; 100% (185) of the residents said that the output and its own features play a key role in disease progression and the management plan that should be followed.
Regarding pain in the ipsilateral region associated with CDS followed by cracked skin and cellular tissue, 91.8% (170) of respondents said that this is something that should be paid attention to with further follow-up because it can become a true complication while 8.2% (15) said it is a normal finding after CDS placement.
DISCUSSION
In the results reported, it is surprising to see the approach that residents who responded to the survey would take. Therefore, 94% would place a CDS in a patient with empyema while 6% would refuse to do so. Although it is a minority, if we extrapolate it to a much larger population, the number becomes much bigger too. A study conducted in Venezuela reported success rates in 64.59% of the cases in advanced stages of empyema without requiring any other approach while 35.41% of the cases required a CDS using the closed technique right from the start. However, these patients had an unfavorable progression, and surgery was eventually needed8. Therefore, we can deduce that the first-line therapy for the management of empyema should be to drain it and then proceed with CDS implantation to eventually assess if the patient can benefit from another surgical act.
A total of 86.5% of respondents would place a CDS for the treatment of hemothorax. The first-line therapy here would be to confirm the actual etiology, stabilize the patient if needed, and quantify whether the hemothorax is < 300 cc. With stable clinical signs we could proceed with clinical-radiographic monitorization. If > 300 cc and/or hemodynamically unstable patients should be implanted with a 28-Fr-to-36-Fr pleural drainage tube9,10.
Regarding tension pneumothorax, 86.5% of respondents would place a CDS while 45.9% would do so to treat a grade 2 pneumothorax. None of the respondents would place a CDS for the treatment of minimum pneumothorax while only 1.6% would decide to place a CDS to treat a pneumothorax with clinical repercussions. The management of pneumothoraxes can go from conservative treatments all the way up to pleural resection depending on the cause, intensity of pneumothorax, the patient’s symptoms, and associated conditions11. In the management of tension pneumothoraxes, the first-line approach is drainage by puncturing the second intercostal space in the midclavicular line. Therefore, after achieving clinical stability, a drainage tube should be placed. Interrogating the patient is of paramount importance to know the cause and past episodes to then contemplate the possibility of performing further surgical acts12,13. Another surprising piece of information is the scarce number of respondent residents who would decide not to drain the pleural cavity when the pneumothorax causes some kind of clinical repercussions regardless of the amount of air found in the cavity.
Regarding chylothoraxes, 49.7% of respondents agrees that one of the pillars for the management of chylothoraxes, based on a study conducted in the United States back in 2009, is to establish a guideline for management and treatment purposes. It is obvious that treatment is something much more complex based not only on pleural space drainage, but also on nutritional support. At the early stage, causes should be studied thoroughly. Also, assess whether further surgical treatment will be required based on a series of criteria already established being pleural output quantified in 24 hours or 5 consecutive days if persistent for over 2 weeks and in the presence, or not, of nutritional-metabolic compromise14.
Almost all patients with pleural effusion should be treated with diagnostic thoracocentesis unless there is little pleural fluid. If the presence of transudate is confirmed, the underlying cause should be treated and if the patient shows clinical signs, an evacuation thoracocentesis15 should be suggested15, which is why 7.4% of respondents delayed this approach when suggesting a CDS as the first-line treatment. A total of 1.6% of participants said they would place a CDS for the treatment of parapneumonic and/or partitioned effusions. There is no doubt that the existence of an intrapleural hydro-aerial level on the chest x-ray in a pneumonia setting is also an absolute indication for drainage of a parapneumonic effusion preferably using 8-Fr-to-14-Fr drainage tubes in non-purulent complex pleural effusions. In the absence of hydro-aerial level, non-purulent effusions should be treated with evacuation throracocentesis. Also, it should be assessed whether it loculates well or reaccumulates and whether, during the index throracocentesis, characteristics indicative of possible poor progression were seen (Gram staining or positive culture; pH 1000 IU/L), which would be indicative of the use of a pleural drainage tube without mentioning that in loculated effusions such loculations should be broken to achieve the complete drainage of pleural cavity by instilling fibrinolytic agents through the thoracotomy tube (eg, 100 000 units of urokinase dissolved in 100 mL/day of physiological serum). If conservative treatment fails, thoracoscopy drainage can be attempted15.
Malignant pleural drainage is often performed in the malignant neoplasm setting and can be a direct consequence of such process or be indirectly associated with it (in this case, it is known as paraneoplastic effusion)16. A total of 9.8% and 7.5% of residents surveyed said that a CDS should be placed for the treatment of malignant pleural drainage or paraneoplastic effusion, respectively. The therapeutic goal, in these cases, is just palliative care to give the terminally ill patient the best possible quality of life17. The management of these effusions depends on factors like the triggering symptoms, the patient’s functional status, the type of tumor and response to different treatments, and the possibility of spreading again towards the lung after evacuation thoracocentesis. The most common therapies of all are repeated evacuation thoracocentesis, and pleurodesis through thoracostomy or thoracoscopy when there are no chances of expansion to the lung but there’s an increased fibrinolytic activity. However, neither one of these techniques is comfortable for terminally ill patients since they require hospital admission or repeated punctures. Therefore, other techniques have been designed for outpatient and continuous drainage like the placement of a pleural drainage tube tunneled in continuity18.
Regarding what we already know of pleural drainages, we confirmed that less than half of the respondents (48%) barely know anything about the indication that the tube should always be submerged 2 cm below water level. Also, 5.8% think that the bottom of the water container should be removed as many times as necessary to stay at this measurement since, if during respiration, the patient creates intrapleural pressure of < 20 cm of water, then the water level of the seal goes up 1 cm through the tube, thus leaving another centimeter for safety at water level19. A total of 3.8% say that estimating the pleural output through the CDS is not necessary when 1 of the criteria for drainage is pleural outputs < 200 mL within 24 hours20.
CONCLUSION
A total of 94% would place a CDS in a patient with empyema while 86.5% would do so for the treatment of hemothorax. A total of 86.5% of respondents would place a CDS to treat tension pneumothorax, 45.9% would do so for the treatment of grade 2 pneumothorax while 53.5% would make the decision of placing a CDS to treat chylothorax.
A total of 49.7%, 23.2%, 9.7%, 7.5%, and 1.6% of respondents would place a CDS for the treatment of pleural effusion with characteristics of exudate, malignant pleural effusion, paraneoplastic effusion, pleural effusion with characteristics of transudate, and pneumothorax with clinical repercussions, respectively, in both parapneumonic and partitioned pleural effusions. None of the respondents would place a CDS for the treatment of minimum pneumothorax.
Regarding know-how on the management of pleural drainage system, only 48% of respondents was right on the baseline level to which drainage tube should be kept sealed while only 21% of participants answered correctly that the bottom of the water container should be removed depending on the CDS pleural output. A total of 96.2% answered correctly that it is necessary to quantify the CDS pleural output while 100% agreed that it was necessary to control the output and its capacity to affect its progression and plan that should be followed. A total of 98.1% of respondents are aware of the complications that can occur after placing a CDS.
A total of 51.9% felt, at one time or another, the need for placing a CDS.
When we review the indications for CDS placement and postoperative care there is still controversy on the knowledge we have accumulated thus far.
REFERENCES
1. Victoria V, Garrido P. Enfermedades de la Pleura [Internet]. Madrid: ERGON. 2003. Disponible en: https://www.neumomadrid.org/wp-content/uploads/monog_neumomadrid_v.pdf [ Links ]
2. Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis [Internet]2021. 2020; 20: 425-34. Disponible en: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30086-4/fulltext [ Links ]
3. Guijarro R, Cantó A. Historia del drenaje torácico. Arch Bronconeumol[Internet] 2002 [consultado en el 2021]; 38(10): 489-91. Disponible en: Disponible en: https://www.archbronconeumol.org/es-pdf-S030028960275271X [ Links ]
4. Tapias L, Vargas L. Complicaciones de los tubos de tórax. Rev Colomb Cir[internet]. 2009 [consultado 2021] [ Links ]
5. Millikan J, Moore E, Steiner E, Aragon G. Complications of tube thoracostomy for acute trauma. Am J Surg [internet]. 1980 [consultado 2021]. 140(6):738-41. Disponible en: Disponible en: https://pubmed.ncbi.nlm.nih.gov/7457693/ [ Links ]
6. Baily C. Complications of tube thoracostomy in trauma. J Accid Emerg Med [internet]. 2000 [ consultado 2021]. 17: 111-114. Disponible en: https://emj.bmj.com/content/emermed/17/2/111.full.pdf [ Links ]
7. Chad G, Jason L, Kevin B. Chest tube complications: How well are we training our residents?. J can chir [internet]. 2007 [ consultado 2021]. 50 (6) 450-458. Disponible en: Disponible en: https://www.canjsurg.ca/content/50/6/450.long [ Links ]
8. Molina J, Guzmán F. Conducta terapéutica en el empiema torácico. Actual infectología [internet]. 2000 [consultado noviembre 2021]. 16 (3): 12-20. Disponible en: https://pesquisa.bvsalud.org/portal/resource/pt/lil-310634 [ Links ]
9. Garrido V, Sancho J, Blasco L. Diagnóstico y tratamiento del derrame pleural. Arch Bronconeumol [internet]. 2006 [consultado en noviembre 2021].42(7):349-72 Disponible en: Disponible en: https://www.archbronconeumol.org/es-pdf-S0300289606706652 [ Links ]
10. Light R. Pleural controversy: Optimal chest tube size for drainage. Respirology [Internet]. 2011 [consultado en noviembre 2021]. 16, 244-248. Disponible en: Disponible en: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1440-1843.2010.01913.x [ Links ]
11. García G. Terapia médica en urgencias. Urgencias en patología pleural. 5th ed. México. Editorial Panamericana. 2015. [ Links ]
12. Borja A, Aranda J, Busca P. Guía de práctica clínica de la SECT sobre el manejo de pacientes con neumotórax espontáneo. Revist de cirugia española [internet]. 2017 [consultado en noviembre 2021]. Vol. 96. Núm. 1. páginas 3-11. Disponible en: Disponible en: https://www.elsevier.es/es-revista-cirugia-espanola-36-articulo-guia-practica-clinica-sect-sobre-S0009739X17302798 [ Links ]
13. Thomas G, Brims F. P35 Primary spontaneous pneumothorax: adherence to guidelines and healthcare economic implications. Thorax [internet]. 2011 [consultado en noviembre 2021] 66. A81-A82. Disponible en: Disponible en: https://thorax.bmj.com/content/66/Suppl_4/A81.3 [ Links ]
14. Emmet M, Zoe A. Chylothorax: Aetiology, diagnosis and therapeutic options. Respiratory Medicine[internet]. 2010 [consultado en noviembre 2021]. 104, 1e8. Disponible en: Disponible en: https://www.resmedjournal.com/action/showPdf?pii=S0954-6111%2809%2900258-3 [ Links ]
15. Porcel M. Manejo práctico del derrame pleural. An. Med. Interna (Madrid) [Internet]. 2002 [consultado 2021 Nov]. 19(4): 58-64. Disponible en: Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-71992002000400011&lng=es . [ Links ]
16. BArbetakis N, Asteriou C, Papadopoulou F, [et all]. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg. [internet]. 2010 [consultado en noviembre 2021]. 5: 27. Disponible en: Disponible en: https://cardiothoracicsurgery.biomedcentral.com/articles/10.1186/1749-8090-5-27 [ Links ]
17. Giangreco M, Canale M. Tratamiento del derrame pleural maligno con pleurodesis química. Rev. Cir. Parag. [internet]. 2012[ consultado en noviembre del 2021]. Vol. 36; Nº 2. Disponible en: Disponible en: http://scielo.iics.una.py/pdf/sopaci/v36n2/v36n2a04.pdf [ Links ]
18. Pérez C. Drenaje tunelizado para el tratamiento ambulatorio del derrame pleural recidivante. Patología Respirator[internet]. 2013 [consultado en noviembre 2021]. Vol 16, supl 1. Disponible en: Disponible en: https://www.revistadepatologiarespiratoria.org/descargas/pr_16-s1_s62-s64.pdf [ Links ]
19. Velásquez M. Manejo De Los Sistemas De Drenaje Pleural. Rev Colomb Cir[internet]. 2015. [ consultado en noviembre 2021]. 30, 131-138. Disponible en: Disponible en: https://www.revistacirugia.org/index.php/cirugia/article/view/339 [ Links ]
20. Younes N, Gross L, Aguiar S, Haddad J. When to remove a chest tube? A randomized study with subsequent prospective consecutive validation. Journal of the American College of Surgeons [internet]. 2002[consultado en noviembre de 2021] 195(5). Disponible en: Disponible en: https://www.journalacs.org/article/S1072-7515(02)01332-7/fulltext [ Links ]
Received: July 19, 2022; Accepted: February 02, 2023










 texto en
texto en