Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Cirugía paraguaya
versão On-line ISSN 2307-0420
Cir. parag. vol.46 no.3 Asunción dez. 2022
https://doi.org/10.18004/sopaci.2022.diciembre.12
Original article
Prevalence of complications post insertion of Port-a-Cath® in the Hospital Central de Instituto de Previsión Social, 2019 to 2020
1Instituto de Previsión Social, Hospital Central. Asunción, Paraguay
Introducción: Los dispositivos tipo Port-a-Cath® generalmente se indican en pacientes oncológicos con tratamiento quimioterápico prolongado, antibioticoterapia, transfusiones de componentes sanguíneos. Igualmente pueden ocurrir complicaciones relacionadas al catéter que pueden llevar a su mal funcionamiento. El objetivo de esta investigación es estudiar la frecuencia de las complicaciones relacionadas con la colocación de los mismos. Métodos: Se realizó un estudio tipo observacional descriptivo retrospectivo. Se estudiaron un total de 337 pacientes con colocación de Port-a-Cath® en el Hospital Central del Instituto de Previsión Social del año 2019-2020. Resultados: Corresponden al sexo masculino 106 (32%) y al sexo femenino 231 (68%). Se obtuvo una frecuencia de complicaciones de 4,7%. Entre las complicaciones más frecuentes se informan: infección en un 2,6%, trombosis del catéter en 0,8%. Conclusión: Los accesos venosos tipo Port-a-Cath® son implantes permanentes que presentan una baja incidencia de complicaciones, entre las más frecuentes encontramos trombosis e infección.
Palabras clave: Port-a-Cath®; complicaciones; quimioterapia; infección; trombosis
Introduction: Port-a-Cath® type devices are generally indicated for cancer patients with prolonged chemotherapy treatment, antibiotic therapy, transfusions of blood components. Complications associated with the catheter can also occur and lead to its malfunction. The objective of this research is to study the frequency of complications associated with their placement. Methods: A retrospective descriptive, observational study was conducted. A total of 337 patients with Port to Cath placement at Central Hospital of the Social Security Institute, Asunción, Paraguay from 2019 through 2020 were studied. Results: 106 patients (32%) were men and 231 (68%) were women. These were among the most common complications reported: infections in 2.6% and catheter thrombosis in 0.8%. Conclusion: The Port to Cath type venous accesses are permanent implants with low rates of complications being thrombosis and infection among the most common of all.
Keywords: Port-a-Cath®. Complications; Chemotherapy; Infection; Thrombosis
Introduction
Currently, subcutaneous venous ports or Port-a-Cath® consist of a tunneled central venous catheter aimed at a subcutaneous pocket where a reservoir or sealed port is implanted. It consists of a port that is inserted into the thoracic wall and a catheter aimed at the junction of the superior vena cava withthe right atrium1,2.
The acquisition of these permanent vascular accesses is, to this date, of paramount importance for the management of patients treated with prolonged IVtreatment. Since these catheters started being used, the administration of chemotherapeutic therapies in cancer patients has become a safer and easier-to-use technique compared to former peripheral and transient systems that had several collateral defects like tissue irritation and sclerosis of vascular endothelium3,4,5.
The possibility of multiple long-term injectionsis among the benefits these devices bring. In addition, they allow us to draw blood in a less painful way. They contribute to improving the patient’s quality of life, do not stop patients from doing the basic activities of daily life, and cosmetically, they are widely accepted. It has been reported that the length of stay (LoS) of the patients is also shorter, which reduces cost6.
Indications for use in cancer patients include chemotherapy, antibiotic therapy, and blood transfusions, resuscitation fluid therapy or access to blood flow for monitoring purposes. On the other hand, performing multiple catheterizations can trigger venous systemthrombosis7,8. Eventually, the use of these subcutaneous ports can reduce the anxiety associated with repeat punctures. Advantages are that these devicesare easy to insert, remove, and handle. In addition, that they can reduce the risk of infection since the skin acts as a natural barrier to microorganisms. However, this technique is no stranger to complications and incidence rate is somewhere between 2% and 14%9,10,11.
Studies conducted reveal that the use of a Port-a-Cath® vs a central venous catheter guarantees treatment continuity in both the mid- and long-term, thus avoiding any interruptions of use12,13,14.
The objective of this studyis to describe the prevalence of complications after placing a Port-a-Cath® at Hospital Central de Instituto de Previsión Social, Asunción, Paraguay from 2019 through 2020.
Materials and methods
This is a retrospective, descriptive, cross-sectional, and observational clinical trial with non-probability sampling of cancer patients with an indication for chemotherapy treated with fully implantable Port-a-Cath® devices at Hospital Central de Instituto de Previsión Social, Asunción, Paraguay from 2019 through 2020.Patients > 18 years-old from both sexes were included in the study. Review data were obtained from the registries of surgical reports and past medical histories ofpatients who underwent such procedure. To study the variables, data were included in a Microsoft Office Excel 2007 spreadsheet for statistical analysis. This study respected the right to privacy and identity confidentiality of the medical records of patients who participated in the study.
Results
A total of 337 patients treated with Port-a-Cath® devices at Hospital Central de Instituto de Previsión Social, Asunción, Paraguay from 2019 through 2020.Patients were studied. A total of 106 were men (32%) and 231 were women (68%) with a mean age of 57 years. A total of 88% and 12% of the cases were treatedat the vascular surgery unit and general surgery unit, respectively.
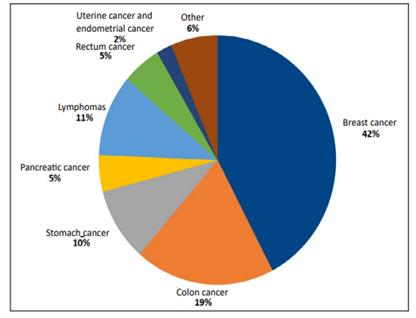
These are some of the conditions—shown by order of frequency—that triggered the use of Port-a-Cath® devices: breast cancer (40%), color cancer (18%), lymphomas (10%), stomach cancer (9%), rectum cancer (5%), pancreatic cancer (4.5%), uterine cancer (2%), neuroendocrine tumor (1%), liver cancer (1%), gallbladder cancer (1%), lung cancer (1%), esophageal cancer (1%), and ampulla of Vater carcinoma (0.5%). (Figure 1).
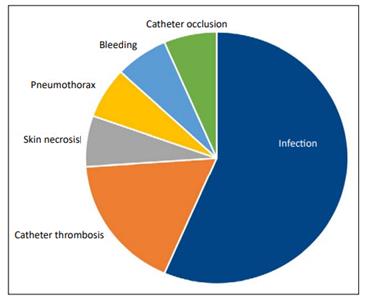
An overall rate of infections of 4.7% was obtained from the total number of cases studies throughout this period was reported. When such complications were detailed the most common ones were infection, 2.6%; catheter thrombosis, 0.8%; skin necrosis, 0.3%; pneumothorax, 0.3%; bleeding, 0.3%; and catheter occlusion, 0.3%. Catheter thrombosis was solved through drug therapy (Figure 2).
Discussion
Regarding the results shown in our new research, we can say that we are within a reasonable range of complications.
Compared to other studies like Koch et al.’s series (1996) of1000 patients or Woloster et al.’s series (2004) of519 patients (withrates of complications somewhere between 1% and 7%, respectively) our rateof 4.7% does not seem that far from these numbers3.
Also, we found series like Brothes et al.’s (1988) that revealed a rate of up to 4.9% of thrombotic events in a group of 329 patients. In our group we found a rate of catheter thrombosis of 0.8% most of which resolved with drug therapy3.
In studies like the one conducted by Kock et al. (1996), the rate of infections was 4.9%. However, this study also reported a rate of infections of 2.6%3.
In series like Johnson’s the rates of pneumothorax and bleeding were somewhere between 0%-1.9% and 0%-3.6%, respectively.11 In our study we found lower rates of pneumothorax and bleeding of 0.3% and 0.3%, respectively.
Conclusion
We have come to the conclusion that Port-a-Cath® venous access devices are permanent implants that are associated with a low rate of complications. Some of the most common ones are thrombosis and infection, which also have a low rate of occurrence.
Continuing medical education for both patient and healthcare team is associated with optimal care and, therefore, with the proper functioning of the Port-a-Cath® venous system. These devices are an excellent alternative to prolonged and repeated therapies in cancer patients due to their versatility, outpatient use, and quality of life they for the patients.
Indications, material selection, type of treatment, complications, and the cost-benefit ratio should be taken into consideration to improve the patient’s quality of life.
REFERENCES
1. Mayoral V, Wong S, Guirola J, Mainar A. Puertos venosos subcutáneos. Principales complicaciones, diagnóstico y manejo. Rev Intervencionismo. 2017; 17(4): 120-9 [ Links ]
2. Silberzwieg JE, Sacks D, Khorsandi AS, Bakal CW, Society of Interventional Radiology Technology Assessment Committee. Reporting standards for central venous acces. J VascInterv Radiol. 2003;14: S443-52 [ Links ]
3. Freire E, De la Iglesia A, Rodríguez C , López M , González M , Peleteiro R , Camba M. Reservorios venosos centrales totalmente implantables, tipo Port-a-Cath(r), en pacientes oncológicos: Revisión de Complicaciones. Rev. Soc. Esp. Dolor. 2008. 7: 451-462 [ Links ]
4. Broviac JW, Cole JJ, Scribner BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet. 1973; 136 (4): 602. [ Links ]
5. Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979; 148 (6): 871-5. [ Links ]
6. Aldrighetti L, Paganelli M, Arru M, et al. Complications of blind placement technique in 980 subcutaneous infusion ports. J Vasc Access 2000; 1: 28-32. [ Links ]
7. El Hammoumi M, El Ouazni M, Arsalane A, El Oueriachi F, Mansouri H, Kabiri EH. Incidents and complications of permanent venous central acces systems: a series of 1,460 cases. Korean J Thorac Cardiovasc Surg. 2014;47:117-23 [ Links ]
8. Narducci F, Jean-Laurent M, Boulanger L, Bédoui E, Mallet Y, Houpeau J, et al. Totally implantable venous acces port systems and risk factors for complications: A one year prospective study in a cáncercentre. Eur J Surg Oncol. 2011;37:913-8 [ Links ]
9. Granziera E, Scarpa M, Ciccarese A, Filip B, Cagol M, Manfredi V, et al. Totally implantable venous access devices: retrospective analysis of different insertion techniques and predictors of complicatios in 796 devices implanted in a single institution. BMC Surgery. 2014;14:27 [ Links ]
10. Araújo C, Silva JP, Antunes P, Fernandes JM, Dias C, Pereira H, et al. A comparative study between two central veins for the introduction of totally implantable venous acces devices in 1201 cancer patients. Eur J Surg Oncol. 2008;34:222-6 [ Links ]
11. Gerson R, Rojas G, Serrano A, Flores R, Villalobos A. Complicaciones asociadas a cateter Port-a-Cath(r). Revista Médica del Hospital General.1998. Vol 61(1): 14-18 [ Links ]
12. Ragusa M, Alberti D, Argento R, et al. Central venous access systems in the oncology patien. Minerva Chir 2000; 55: 139-46. [ Links ]
13. Frezza A, Tommasino U, Festa G, et al. Terapia infusiva a lungo termine: S.A.V.C.T.I. utilizzoindispensabile. Atti del 102º. Congresso della Societá Italiana di Chirurgia (S.I.C). [ Links ]
14. Calvo R, Ruiz J, Rubio V, Belmonte M, Ruz M, Lluch M. Reservorios subcutáneos venosos centrales permanentes. Complicaciones. Rev. Soc. Esp. Dolor. 2004; 11(3) : 50-55 [ Links ]
4Authors’ contributions: All the authors contributed equally to develop the protocol, its application, and drafting of final report, and corrections.
Received: October 10, 2022; Accepted: November 08, 2022










 texto em
texto em