INTRODUCTION
Alpaca (Vicugna pacos), is one of the South American camelids of Peru. The main reproductive characteristics of the alpaca include induced ovulation by mating, very high embryo mortality rate and an 11-month gestation period1. The ovaries are oval in shape, and 1.5 to 2.5 cm long and 1.0 to 1.2 cm high in size2. The follicular waves, normally present, last between 9 and 22 days. Spontaneous corpus luteum (CL) formation is rare, and only 5% of the alpacas spontaneously ovulate3.
The preantral follicles represent the main ovarian reserve. Their activation and arrest stages during folliculogenesis are poorly understood4. Considering the significance of the preantral follicles in assisted reproductive techniques, several studies have been conducted to describe their morphometric aspects in cow, goats, ewe and llamas5-8. However, to the best of our knowledge, no reports are yet available regarding the ovarian preantral follicles in the alpaca. Establishing the follicular population of the ovary of adult alpacas could help us to see the possibility of using it as a biological model for studies of folliculogenesis in humans.
The aim of this study was to characterize the preantral follicle population in the alpaca ovary, investigating the following endpoints: follicular morphology (normal vs degenerated), proportion of the primordial, transitional, primary and secondary follicles, as well as the diameters of the follicle and oocyte.
MATERIALS AND METHODS
Animals and ovaries
The methodology, code 102007, was approved by the Institutional Ethics Committee for the Use of Animals (CIEA) of the Universidad Peruana Cayetano Heredia. Ten ovaries from five non-pregnant adult alpacas (2 to 3 years old), with a previous partum, were obtained via median laparotomy cranial to mammary gland, using general anesthesia protocol with Xylazine (1mg/Kg IM), Tramadol (1mg/kg IM) and Ketamine 10% (5mg/kg EV). The ovarian cortical samples were cut into fragments of approximately 3 x 3 x 1 mm sized fragments.
Histological processing
Once harvested, the ovarian tissue was fixed in paraformaldehyde solution at 4 °C for 4 h; then it was placed in 70% alcohol for 24 hours6 and submitted to histological analysis. The ovarian fragments were dehydrated by passing them through a graded series of alcohol, embedded in paraffin wax for 4 hours at 58 °C, and then sectioned serially into 7 μm thick slices. The samples were stained using Harris hematoxylin and eosin.
Follicle morphology and development
The histological samples were analyzed using LEICA light microscopy (Wetzlar, Germany) at 400x magnification. The histological evaluation and classification of the follicles were done by a single operator. Based on their morphology, the follicles were categorized as normal (follicle containing an intact oocyte and granulosa cells, well organized in layers, without pyknotic nucleus) or degenerated (oocyte with pyknotic nucleus and retracted cytoplasm, or disorganized granulosa cells detached from the basement membrane)9,10. Every follicle containing a visible oocyte nucleus was examined in each section where it appeared in and matched with the identical follicle on the adjacent sections to prevent double counting. The follicle and oocyte diameters were measured only in the morphologically normal follicles, using the LEICA software coupled with a LEICA light microscope. The follicle diameter was measured from one edge of the outermost granulosa cell layer to the opposite edge. Oocyte diameter was measured from one edge of the oocyte membrane to the other. Two perpendicular diameters were recorded for each measurement, and the average of both those values was calculated. Based on their developmental stages the morphologically normal preantral follicles were classified as primordial and developing follicles (transitional, primary and secondary), as reported by Silva-Santos et al.12.
Statistical analysis
The number of follicles at all stages, primordial and developing (transitional, primary, or secondary), were expressed as mean + SD. The diameters of the primordial, transitional, primary and secondary follicles, and oocytes were analyzed using the analysis of variance (ANOVA), and Duncan’s mean test.
RESULTS
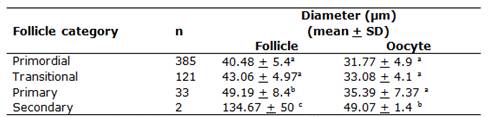
A total of 624 preantral follicles were evaluated. The normal and degenerated follicles are shown in Figure 1. The overall percentage of the normal preantral follicles was 86.70%. The preantral follicle population showed the following distribution: 71.16% primordial, 22.37% transitional, 6.10% primary, and 0.37% secondary. The follicular and oocyte diameters are shown in Table 1. The primordial and transitional follicles showed similar diameters, which progressively and significantly increased, until they became secondary follicles. The largest oocyte diameter was found in the secondary follicles (P < 0.05).
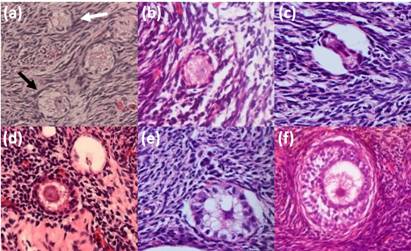
Figure 1: Photomicrographs of the alpaca preantral follicles depicting normal (black arrow) and degenerated (white arrow) primordial follicles (a) normal (b) and degenerated (c) transitional follicles; normal (d) and degenerated (e) primary follicles and normal secondary follicles (f) (magnification 400X).
DISCUSSION
This study is the first to report the histologic evaluation of the preantral follicles of the ovaries of adult alpaca. The occurrence of normal follicles was 86.72%, which concurs with the earlier reports for goats; (91.7%)(13).
The proportions of primordial, transitional, primary and secondary follicles were determined in the present study. Interestingly, the percentages of primordial (71.23%) and transitional follicles in alpaca were similar to those reported for small ruminants (goat(6) and ewe(7)), while the proportion of the primary follicles (6.05%) corresponded to the findings in bovines(5). Also, Arias et al.(8), in their analysis of ovarian samples of the llama species (Lama glama), found that most preantral follicles were in the primordial state.
The diameter of the alpaca primordial follicles (40.48 µm) showed similarity to the other species, such as llama (23 - 49.4 µm;(8)), cow (45.4 µm;(5) and ewe (33 µm;(7)). Alpaca’s primary follicles (49.19 + 8.4 µm) were larger than the primordial and transitional ones, but smaller than the llama’s primary follicles (62.4 to 75.4 µm;(8)). Similar results were recorded for the secondary follicles.
Oocyte diameters varied from 31.77 to 49.07 µm and showed similarity among the primordial, transitional and primary follicles; however, they were significantly different from the secondary follicles. Comparable results for oocyte diameters (31.2 and 49.5 µm, for primordial and secondary follicles, respectively) were also described in cow(5);(4). Nevertheless, for both follicular stages, smaller oocyte diameters were observed in alpacas compared to llamas (90 µm)(8).
In conclusion, this is the first study on the preantral follicle population in the adult alpaca ovarian cortex. From the results, it is evident that most of the preantral follicles in the alpaca ovarian cortex were in the primordial or transitional stages and a low rate of follicle degeneration was observed. Regarding the follicle and oocyte diameters, when compared with the literature available on the preantral follicles of llama, the other south American camelid, smaller follicular (primary and secondary follicles) and oocyte (enclosed in primordial and secondary follicles) diameters were found in alpaca, in the current study. This study serves to establish a biological model for future reproduction studies in the Alpacas. Likewise, it serves as a basis for the use of the alpaca ovary as an artificial ovarian model for the human ovary to analyze in vitro folliculogenesis.