INTRODUCTION
Leishmaniases are distributed worldwide and have been reported in 98 countries, with a global prevalence of 12 million people and a population of 350 million at risk of infection1,2. The diseases are caused by Leishmania Ross, 1903, which in the neotropical region are transmitted to animals and humans by females of Lutzomyia França, 1924 (Psychodidae: Phlebotominae). The main clinical forms found in the Americas are mucocutaneous (MCL) and cutaneous leishmaniasis (CL), which is endemic to Brazil3. In 2015, an amount of 197,552 new CL cases were reported to the World Health Organization4.
In the State of Paraná, in southern Brazil, indigenous CL transmission has been detected in three regions: the Ribeira de Iguape River Valley (east), where the disease has been reported for more than a century5; in northern Brazil, where an outbreak was reported in 19946-8 and in the central region, where reports have been reported since 20029. In the same State, the parasite isolated from humans and dogs in the different regions was Leishmania (Viannia) braziliensis Vianna, 19118-11. The vector Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) was found infected with the same species in the northern part of the State7. In this region, L. (Viannia) was also detected in Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912)12,13. Only one wild animal species (Nectomys sp.) has been verified with infection by L. braziliensis10.
CL has been reported in humans in the Ribeira Valley, which comprises a part of the State of Paraná and another part of the State of São Paulo, since the 1950s14,15. In the 1980s, Gomes and Galati(16,17) studied the sand flies of Ribeira Valley (São Paulo State side), and found a very diverse fauna. In 1987, the authors reported 13 species with a predominance of L. intermedia (77.9%), suggesting that this species might be better suited to occupy vacant ecological niches or anthropogenic alterations. In 1989, the authors observed 19 species with a predominance of Lutzomyia (Psychodopygus) ayrozai (Barretto & Coutinho, 1940) (67.5%) in the soil of forest. In this region, L. intermedia is widespread5,18-20. In this taxon, the existence of a complex of species or lines (L. intermedia sensu lato) has been proposed, formed by Lutzomyia intermedia sensu strictu and Lutzomyia (Nyssomyia) neivai (Pinto, 1926), and their differentiation is based mainly on the genitalia morphology of either male or female insects21,22. Then, one wonders which lineage(s) is (are) present in the Ribeira Valley region where CL remains endemic. Whether the sand flies described in this region remain diversified or only some species have reportedly occupied vacant niches that have been unveiled by human activity and whether the Phlebotominae fauna could be changed by the Bolivia-Brazil gas pipeline construction (1998-2009) in this area, resembling the human CL case reported by Castro et al.5. This work analysed the fauna of sand flies in two municipalities of Ribeira Valley (Adrianópolis endemic area and Cerro Azul with outbreak area). It is necessary to evaluate what has happened in the Ribeira Valley region regarding the adaptation of Leishmania vector species and will help to clarify the epidemiological role of L. intermedia and other species in the Leishmania cycle.
MATERIAL AND METHODS
Description of the study area
The State of Paraná is located in the southern region of Brazil, between latitudes 22º30'58" and 26º43'00" South, and between longitudes 48º05'37" and 54º37'08" West. Its landform shows moderate altitudes, with three distinct topographic units: plain, escarpment, and plateau regions. The first comprises the Littoral Plain, and the second corresponds to the Atlantic Massif, divided into Serra do Mar and Serra Geral; the latter is subdivided into the Serra Geral Oriental. The third unit comprises the plateaus of Campos Gerais, Guarapuava, and the Crystalline Atlantic Plateau, in which Ribeira River is located23.
Sand flies collection was performed in the municipalities of Cerro Azul and Adrianópolis (Figure 1). The total area of Cerro Azul (located at latitude 24º49’25” South and longitude 49º15’40” West, altitude 393 meters) is 1,341 km2, with a population of 17,725 estimated for 2018. Adrianópolis (latitude 24º39’26” South, longitude 48º59’28” West, altitude 154 meters) has 5,983 inhabitants (estimated value for 2018) distributed over an area of 1,349 km2(24.
Sand flies collection
Collections were conducted using Communicable Diseases Center (CDC) type light traps25, in the months of December (1 night) 2008 and February (3 nights), May (1 night), and November (1 night) 2009. The collection was done between 7 PM and midnight. The ecotopes where these traps were installed were the domiciliary environment, the peridomicile, and the woods. The Phlebotominae sand flies were separated by sex, under a stereoscopic microscope. The males were stored in tubes containing 70% ethanol prior to identification. All females were dissected for identification based on the morphology of the spermathecae by pressing a cover slip over the microscope slide that contained the end portion of the sand fly abdomen, immersed in sterile 0.9% saline solution, and observed at 400x magnification. Under a stereoscopic microscope, and in sterile microscope slides, the males were immersed in 0.9% saline solution to determine the size of the palpi, and dissection of the abdomen (4 last segments) was performed for morphological preparation. The end portion of the abdomen was maintained in plates containing 20% KOH for 12 h, until clarification was achieved. Then, dimensions and morphological features of the male genital structures were analyzed, and the species were identified and classified according to Young and Duncan26. In this study, all the captured females belong to L. intermedia, with spermathecae possessing over 11 rings, and have been identified as belonging to the lineage L. intermedia s.s. The males and other females have been associated with the lineage L. intermedia s.l.
After transportation from the field to the laboratory, the females were anesthetized with chloroform and placed on a sterile microscope blade with one drop of sterile 0.9% saline solution. The heads, wings, and legs of the specimens were separated from the thorax and abdomen. Extraction of the gastrointestinal tract was performed with blades (sterile needles) placed on the thorax and the last two abdominal segments. The digestive structures (foregut, midgut, hindgut, proventriculus, and pharynx) were dissected to verify the presence of flagellates.
Statistical analysis
The BioEstat 5.0 statistics package27 and Excel software (Microsoft Office 2010) were used to perform the Chi square test, Haberman’s standardized residuals test, and theZ-test for 2 proportions, mutually exclusive categories, for the amount of specimens from the taxon L. intermedia (subgenus Nyssomyia) collected from both sexes and from each ecotope. The results were considered significant when p<0.05. The Z-test for two proportions (mutually exclusive categories) was also applied by using the SigmaStat 3.5 package28, on the amount of specimens from each lineage of the taxon L. intermedia collected, as a function of the ecotope of origin. This test was likewise applied to the subgenus Pintomyia, to the total amount of specimens from the species Lutzomyia (Pintomyia) fischeri (Pinto, 1926) in relation to the amount of specimens from the species Lutzomyia (Pintomyia) pessoai (Coutinho & Barretto, 1940).
RESULTS
Collected Phlebotominae sand flies
A total of 432 specimens (304 males, 128 females) were collected: 114 in the woods, 107 in the peridomicile and 211 from the domiciliary environment. The species identified were: L. intermedia (403 specimens, with 219 specimens collected in Adrianópolis and 184 in Cerro Azul), L. fischeri (19 specimens, with 1 specimen collected in Adrianópolis and 18 in Cerro Azul), L. pessoai (6 specimens, all collected in Cerro Azul), Lutzomyia migonei (França, 1920) (1 specimen, collected in Cerro Azul) (Table 1). The prevalence was determined for the taxon L. intermedia, with 219 (50.69% of the total amount of specimens collected) and 184 specimens (42.59% of the total amount of specimens collected) in Adrianópolis and Cerro Azul, respectively. This species was predominant in all ecotopes (Figure 2). The other species corresponded to 0.69 and 6.0% of the total amount in Adrianópolis and Cerro Azul, respectively. Lutzomyia fischeri ranked second, followed by L. pessoai and L. migonei.
Table 1: Percentage of specimens observed in different ecotopes in municipalities of Cerro Azul and Adrianópolis (M=male, F=female)
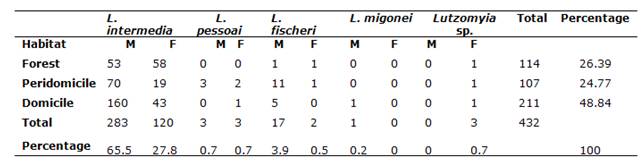
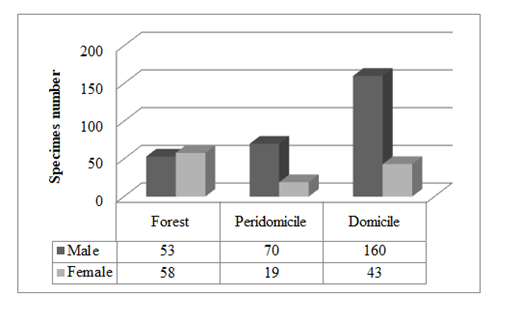
Figure 2: Number of insects by sex and ecotope of Lutzomyia intermedia in Cerro Azul and Adrianópolis
Among the 120 females of the L. intermedia captured, 106 were of L. intermedia s.l., 14 were L. intermedia s.s.; both were present in all the collection environments (Figure 3). Among the 14 females of L. intermedia s.s., 13 were captured in Adrianópolis and one in Cerro Azul.
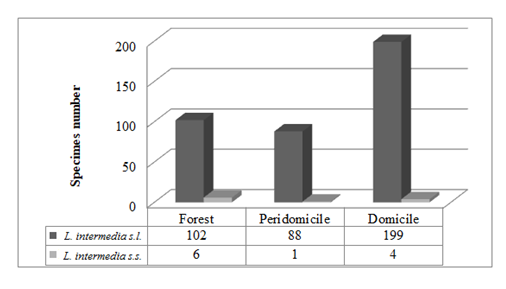
Figure 3: Number of insects per lineage and ecotope of Lutzomyia intermedia in Cerro Azul and Adrianópolis.
The presence of promastigote was not detected in 128 dissected females.
Statistical analysis
The Chi square test, applied to the amount of specimens of each sex of L. intermedia, showed significant differences in all ecotopes (p=0.000), and differences were likewise identified between the domiciliary and peridomiciliary environments (Z-test for two proportions, mutually exclusive categories; p=0.0000) (Table 2).
Table 2: Occurrence of insects of both sexes of Lutzomyia intermedia for each ecotope (M=male, F=female)
| Sex | Domicile* | Peridomicile* | Forest |
| M | 160 (39.7%) | 70 (17.4%) | 53 (13.2%) |
| F | 43 (10.7%) | 19 (4.7%) | 58 (14.4%) |
Chi2 test significant, p<0.0001.
* Statistically significant differences for sex between proportions in ecotopes (Z test for two proportions - mutually exclusive categories; p<0.0001)
The amount of specimens of the lineage L. intermedia s.l. showed a significant difference from the amount of L. intermedia s.s. in all 3 environments (χ2=9.943; DF=2; p=0.07), and Haberman’s residuals test indicated that given the frequencies observed, the number of specimens of the former lineage was below that expected for a wild environment, whereas in the same environment the amount of specimens observed in the lineage L. intermedia s.s. was greater than expected. The Z-test for 2 proportions (mutually exclusive categories) also revealed significant differences between the amounts of specimens of L. intermedia s.l. and L. intermedia s.s. in all ecotopes (p=0.000), as well as significant differences between the amounts of L. fischeri and L. pessoai (p=0.002).
DISCUSSION
In both regions studied (Adrianópolis and Cerro Azul) L. intermedia, L. fischeri, L. pessoai, L. migonei and Lutzomyia sp. (3 specimens) were collected. Statistical analysis applied to the amounts of specimens within subgenera showed significant differences between sand flies of lineages from subgenus Nyssomyia and the subgenus Pintomyia. However, the difference found between L. intermedia s.l. and L. intermedia s.s. was greater than that determined between L. fischeri and L. pessoai, which predominated in the peridomicile environment. Prevalence by ecotope was homogenous between females from both lineages of L. intermedia, both occurring in larger amounts in the wild environment, followed by the domiciliary and peridomiciliary environments. This is a common feature of their distribution among the different environments.
The low densities of the populations of L. fischeri and L. migonei in the Ribeira Valley could indicate the poor capacity of these species to override barriers imposed by artificial environments, as mentioned by Gomes et al.29. However, the participation of these species in the transmission cycle of Leishmania to humans must be considered, particularly when human or domestic animals enter wild environments or secondary forest. Lutzomyia fischeri was observed infected by L. (Viannia) in Porto Alegre (southern Brazil) by Pita-Pereira et al.30 supporting the hypothesis herein proposed. In addition L. migoneiwas implicated in the L. braziliensistransmission in Northeast Brazil and in Rio de Janeiro (southeastern Brazil), where this species was observed naturally infected31,32.
Haberman’s residual test shows that the amount of specimens from the lineage L. intermedia s.s. was even greater than expected, while those from the lineage L. intermedia s.l. resulted in an amount below expectations. In behavioral research conducted on L. intermedia s.s. and L. intermedia s.l. in Iporanga, State of São Paulo, the females of the former were reportedly more attracted to a black-colored Shannon’s trap, which could imply a preference of the lineage for darker, moist locations, represented by areas with dense vegetation, such as a wild environment33.
Lutzomya intermedia (male and female specimens) were prevalent and found in all three environments (domicile, peridomicile and woods). They are predominantly in the domiciliary environment (50.37%). The high prevalence of males in the domiciliary and peridomiciliary environments could reflect the liking behavior of Phlebotominae sand flies in artificial environments in order to establish territories and promote mating. Such locations are usually those frequented by vertebrate hosts34.
The statistical analysis suggested a balance between males and females of L. intermedia in the wild environment. In effect, the majority of the females were collected in the woods (48.33%), suggesting that Leishmania transmission to humans can occur in this environment because it is the habitat where the reservoir mammals live. This kind of transmission has already been reported in regions where the population is accustomed to entering the woods for hunting, fishing, timber, and other forms of extraction5.
In spite of low human population density, actually only secondary forest can be found with grown bananas, beans, rice, maize and cassava cultures. In some areas the native forest has been replaced by Pinus elliotes5. The high prevalence of L. intermedia and the low amount of other sand flies species are in agreement with others studies in the Ribeira Valley5,16-19,29,35. Deforestation clearly has an effect on L. intermedia with respect to the diversity of Phlebotominae sand flies in several areas; where the wild environment remains intact, this species is captured almost accidentally, but after deforestation, this species becomes absolutely dominant playing an important role in L. braziliensis transmission even in a peri-urban region or urban areas as proposed by Gomes and Galati16,17.
Considering the potential role of L. intermedia and other species as vectors for Leishmania, the search for the protozoan in the females of Phlebotominae sand flies is an important strategy to elucidate the transmission cycle of leishmaniasis in the Ribeira Valley region and others endemic regions. The absence of promastigote forms in all 128 dissected females indicates the existence of a low parasitic pressure in the transmission cycle of L. braziliensis in the Ribeira Valley in Paraná. We looked into the isolation of the parasite but not with molecular biology. Oliveira et al. working with molecular approach (multiplex PCR) observed only 0.22% rate of infection by Leishmania sp. in L. intermedia s.l. corroboranting our results12. Indeed, in the region of the work, the parasitic pressure by L. braziliensis in humans is low, with 79 cases from 2007 to 2013 in Adrianópolis county, and 107 in Cerro Azul36. Cerro Azul is an area without CL transmission after the pipeline construction, and had many influences in its landscape due to the passage in this region of the Bolivia-Brazil gas pipeline, which may be associated with a greater diversity of sand flies found. Great impact constructions have been related to changing epidemiological profiles of CL5,37,38.
In summary, we observed a great change in the profile of the sand flies population in relation to the past 30 year’s research in the Ribeira Valley. There is currently almost absolute predominance of L. intermedia s.l. and yet CL remains endemic despite the vectors population profile changes, the environment and the climate. This shows that L. braziliensis presents an ecological plasticity and can adapt to the human environment and possibly adapt to the peri-urban or urban areas as occurred in Belo Horizonte, State of Minas Gerais39, since there is the founder effect chance. The Ribeira Valley could serve as a model for understanding the evolution of the epidemiological chain of L. braziliensis in colonized areas.