Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Memorias del Instituto de Investigaciones en Ciencias de la Salud
On-line version ISSN 1812-9528
Mem. Inst. Investig. Cienc. Salud vol.14 no.2 Asunción Aug. 2016
https://doi.org/10.18004/Mem.iics/1812-9528/2016.014(02)35-039
Articulo Original/ Original Article
Determinación de Aflatoxinas en Maní y productos de Maní en Sudán usando AflaTest® y HPLC
Determination of Aflatoxins in Groundnut and Groundnut products in Sudan using AflaTest® and HPLC
Salah Eldeen Abass Ali AhmedI,II*, Abdalla Ahmed ElbashirI
I University of Khartoum, Faculty of Science, Department of Chemistry, Khartoum, Sudan, P.O. Box 321, 11115
II Environmental Contaminant and Toxic Chemicals Department, National Chemical Laboratories, Federal Ministry of Health, Khartoum, Sudan, P.O. Box 287.
R E S U M E N
Esta encuesta examinó 30 muestras de maní y 15 productos de maní de tres estados (Khartoum, Kordofan y Gadarif) de Sudán para determinar AFB1, AFB2, AFG1, and AFG2 usando cromatografía líquida de alta performance (HPLC) con detección de fluorescencia. La frecuencia de muestras de maní contaminadas con AFB1 de Khartoum, Gadarif y Kordofan fue 58,3%, 57,1%, y 66,7%, respectivamente. Ninguna muestra de maní o producto de maní estaba contaminado con AFG1 o AFG2. El límite de detecciones (LDD) y el límite de cuantificaciones (LDC) estuvieron en un rango de 0,01-0,02 ng g-1 y 0,03-0,05 ng g-1, respectivamente. Algunas muestras de maní contenían concentraciones de AFB1 por encima de los límites regulatorios UE. Las muestras más contaminadas con aflatoxinas fueron las del estado de Kordofan.
Palabras clave: maní; productos de maní; aflatoxinas; HPLC; Sudán.
A B S T R A C T
This survey examined 30 samples of groundnut and 15 groundnut products from three states (Khartoum, Kordofan and Gadarif) of Sudan for AFB1, AFB2, AFG1, and AFG2 using high performance liquid chromatography (HPLC) method with fluorescence detection. The frequency of contaminated groundnut samples with AFB1 from Khartoum, Gadarif and Kordofan state was 58.3%, 57.1%, and 66.7%, respectively. No sample of groundnut or groundnut product was contaminated with AFG1 or AFG2. The limit of detections (LODs) and limit of quantifications (LOQs) were found to be in a range between 0.01-0.02 ng g-1 and 0.03-0.05 ng g-1, respectively. Some groundnut samples contained AFB1 concentrations above the EU regulatory limits. The highest contaminated samples with aflatoxins were found in Kordofan state.
Keywords: groundnut; groundnut products; aflatoxins; HPLC; Sudan.
INTRODUCTION
Mycotoxins are a relatively large, diverse group of naturally occurring, fungal toxins, many of which have been strongly implicated as chemical agents of toxic disease in humans and animals. They are unavoidable contaminants in foods and feeds and are a major problem all over the world. Mycotoxins are mainly produced by certain filamentous fungi belonging toAspergillus,PenicilliumandFusariumgenera. Aflatoxins, ochratoxins, trichothecenes, zearelenone, fumonisins, tremorgenic toxins, and ergot alkaloids are the mycotoxins of greatest agro-economic importance (1).
The Food and Agricultural Organization of the United Nations (FAO) has estimated that up to 25% of the world's food crops are significantly contaminated with mycotoxins (2). Mycotoxins are highly toxic and cause severe intoxications in human and animals, some of them are carcinogens, and the economic damage caused by mycotoxins reaches several billion of dollars every year (3).
The number of mycotoxins known to induce signs of toxicity in mammalian and avian species is unknown and different numbers are suggested. The number exceeds 300 and is steadily increasing. The conditions that promote growth of moulds vary, although high moisture and warm temperatures are responsible for most mould growth on feedstuffs (4,5).
It is evident that due to different production, handling, transport and storage conditions, toxin profiles can differ substantially between areas, localities, seasons and even countries. The consequence of these differences is that worldwide trade of food and feed commodities has also resulted in a worldwide distribution of contaminated materials with different toxin profiles.
Aflatoxins are naturally occurring carcinogenic byproduct of common fungi on grains and other crops, particularly maize and groundnuts. They pose a significant public health risk in many tropical developing countries and are also a barrier to the growth of domestic and international commercial markets for food and feed (6).
Aflatoxins are a group of mycotoxins produced by different species of the genusAspergillus. Naturally occurred aflatoxins include AFB1, AFB2, AFG1, and AFG2. The potential hazards of aflatoxins to human health have led to worldwide monitoring programs of the toxins in various commodities as well as regulatory actions by almost countries around the world. Considering the extremely high carcinogenicity of aflatoxins, most developed nations regulate limits of aflatoxins as low as reasonably achievable. Aflatoxins may contaminate many crops including peanuts, corn, cottonseed oil, Brazil nuts, pistachios, spices, copra (dried coconut) and figs with widespread contamination in hot and humid regions of the world (7).
Aflatoxins are produced byAspergillus flavus which can germinate at moisture levels of 15 to 17% but infection and growth require higher moisture. The aflatoxins are potent liver toxins and most animal species exposed to these mycotoxins show signs of liver disease ranging from acute to chronic. These toxins may be lethal when consumed in large doses. G0enerally, young animals are more susceptible than older ones to the toxic effects of aflatoxins5. It has been pointed out that aflatoxin contamination of feed of food-producing animals can result in residues of ingested aflatoxins or its metabolites in edible tissues such as meat, milk and egg (8).
MATERIAL AND METHODES
Samples
All the samples of groundnut (30) and groundnut products (15) analyzed have been collected randomly from markets of three states in Sudan (Khartoum, Kordofan and Gadarif). The samples size was 0.5-1 kg and were kept at freezer till tested. At the time of analysis samples were brought up to room temperature (9). Extraction and clean up of aflatoxins were done using VICAM method; derivatization and determination were done using AOAC method with some modifications (10).
Chemicals
Methanol (CL chem. Lab) HPLC grade, sodium chloride (CARLO ERBA, MARSEILLE, France), acetonitrile HPLC grade, trifluroacetic acid (TFA), were purchased from (ROMIL, UK), acetic acid were obtained from (Riedel-de Haën,Hannover, Germany), sodium sulphate anhydrous was purchased from Prabhat Chemicals, (Mumbai, India), and AFB1, AFB2, AFG1 and AFG2 were purchased from Immunolab GmbH (Kassel, Germany).
Apparatus
HPLC system consisted of an LC 20AB pump, DGU 20A3 degasser unit, auto sampler (SIL-20A) and fluorescence detector (RF 10AXL) (Shimadzu, Kyoto, Japan). To measure the peak area software, LC Solution Version 1.22 was used.
Extraction and Clean up of Aflatoxins
Twenty five grams of groundnut or groundnut product was transferred to one liter blender jar with 5g of sodium chloride, containing 100 ml methanol and blended for 3 min at high speed. The solution was filtered through 24 cm Whatman No 1. Twenty ml of the extracted solution was taken into a 50 ml centrifuge tube; 30 ml of distilled water was added, then mixed well, and filtered through glass microfiber paper. Twenty five ml of filtrate (equivalent to 1 g of test sample) was collected into a 25 ml graduated cylinder, and preceded immediately with Alfa test IAC chromatography.
The aflatoxins were eluted from the column with 2 ml methanol and evaporated. To derivatize the AFs, 200 μl hexane and 50 μl trifluroacetic acid (TFA) were added. The mixture was shaken vigorously using a Vortex for 30 seconds and left to stand for 5 min; 1.95 ml of acetonitrile: water (1:9) (v/v) was added; the mixture was shaken for 30 seconds and left for 10 min to separate. The lower, aqueous layer was collected using an automatic pipette and then subjected to HPLC analysis. Similarly, a working standard mixture was derivatized.
The HPLC conditions were Supelcosil LC18 column, 150 × 4.6 mm internal diameter (I.D.), 5 micron particle size; oven temperature 30 °C, fluorescence detection at excitation 360 nm and emission 476 nm, mobile phase consisted of acetic acid (0.1%): acetonitrile: methanol (59:14:27) was used. The flow rate of the mobile phase was maintained at 1.0 ml min-1 and the injection volume of sample solution was 20 μl.
RESULTS
Method performance:
The calibration curves, using different concentrations, were generated by plotting different aflatoxins peak areas against the corresponding concentrations of calibration samples. All objectives for linearity validation have been matched: coefficient of correlationr = in range from 0.991 to 0.998 was obtained for all of the analytes, indicating good calibration curves. The limit of detection (LOD) (signal-to-noise ratio=3) was calculated to be 0.017 ng/g for AFB1 and AFG1; and 0.01 ng/g for AFB2 and AFG2. The limit of quantification (LOQ) (signal-to-noise ratio=10) was calculated to be 0.05 ng/g for AFB1and AFG1; and 0.03 ng/g for AFB2 and AFG2.
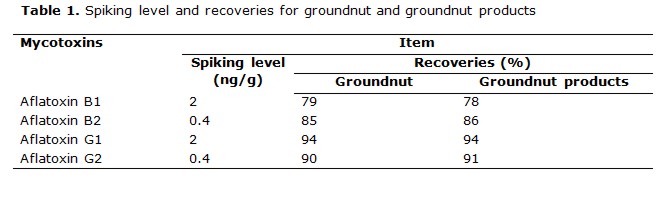
Recovery experiments were determined by spiking AFB1, AFB2, AFG1, and AFG2 at different level of concentrations. The average recoveries of the aflatoxins in range of 73 to 98% , were obtained in table 1 (table 2, table 3) .
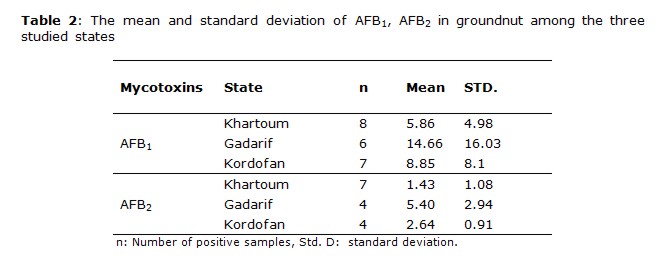
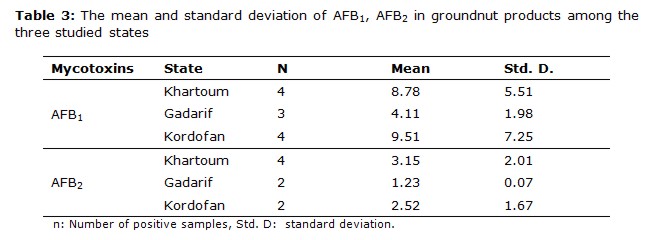
The most frequently found mycotoxin was AFB1 followed by AFB2, this order of the AFB1 and AFB2 is consistent with the results obtained for peanut and peanut products from Sudan (11). Groundnut and groundnut products were highly contaminated with AFB1; its occurrences were 58.9% and 53.3%, respectively, from total samples analyzed. The higher mean values for AFB1 positive contaminated samples were obtained in Kordofan with 17.64 μg kg-1 and 9.51 μg kg-1for groundnut and groundnut products, respectively, in comparison with the other states. The high number of contaminated samples was obtained in Khartoum state.
DISCUSSION
An earlier study carried in Sudan showed that aflatoxin was present in 10-100% of groundnut samples with an average concentration of 8.37±0.61 μg kg-1, they analyzed groundnut samples collected soon after harvest, from different districts in the irrigated region (Central Sudan), and reported that they were free from aflatoxins comparing to other samples collected from the rain fed region (Western Sudan) which showed variable levels of aflatoxin ranging from 100% sample contamination in El Hamdi to only 10% in Casgeal. Other study reported that the groundnut paste exhibited lower aflatoxin contamination than both the gray and the red roasted groundnut and the groundnut products samples collected from Behri reflected a higher contamination than those from Khartoum and Omdurman (12). In other study, 16.6% of groundnut product samples collected from Khartoum state were contaminated with AFG1 and AFG2 (13). In other study 35 samples from 60 samples of stored peanut kernels collected from different cities in Khartoum and Aljazeera states were contaminated with AFB1 with concentration in the range of 17.57- 404.00 μg kg-1 (14). A survey in 1984 was carried out on 228 retail samples of nuts and nut confectionery products comprising peanuts (shelled, unshelled, roasted and salted), mixed nuts, almonds, brazils (in shell), hazelnuts (in shell), chocolate - coated peanuts, peanut brittle and coconut ice. The highest total levels of aflatoxins observed were in unshelled peanuts containing 4920 μg/ kg (15).
The results obtained in the present investigation have shown that mycotoxins contamination in the groundnut and groundnut products is alarmingly high. This is evidently posing a dangerous problem to the poultry and livestock industry as well as to human health.
Furthermore, investigations are recommended to be carried out routinely to study contamination of groundnut and groundnuts product by different types of aflatoxins.
BIBLIOGRAPHICAL REFERENCES
1. Smith JE. Aflatoxins. Handbook of Plant and Fungal Toxicants. New York: CRC Press; 1997. [ Links ]
2. World Health Organization. Basic Food Safety for Health Workers.Geneva:WHO. 1999 [ Links ]
3. Khomutov R, Dzhavakhiya V, Khurs E, Ospova T, Shcherbakova L, Zhemchuzhina N, et al. Chemical regulation of mycotoxin biosynthesis. DokladyBiochmistry and Biophysics. 2011; 1: 25-8. [ Links ]
4. Fink-Grenmels J. Mycotoxins: Their implications for human and animal health. Vet Q. 1999; 21(4):115-20. [ Links ]
5. Crenshaw M. Mycotoxin in Swine Diets. Ergomix Com 2008. [ Links ]
6. Laurian U, Delia G. Aflatoxins - finding solutions for improved food safety. IFPRI, Washington, DC; 2013 [ Links ]
7. Ozer H, Basegmez HO, Ozay G. Mycotoxin risks and toxigenic fungi in date, prune and dried apricot among Mediterranean crops. Phytopathol Mediterr. 2012; 51(1): 148-57. [ Links ]
8. Charoenpornsook K, Kavisarasai P. Mycotoxins in animal feed stuffs of Thailand. KMITL Sci TechnolJ. 2006; 6(1):25 - 8. [ Links ]
9. Richard JL, Bennett GA, Ross PF, Nelson PE. Analysis of naturally occurring mycotoxins in feedstuffs and food. J Anim Sci. 1993; 71: 2563-74. [ Links ]
10. Association of Official Analytical Chemists. Official methods of analysis. Natural poisons; mycotoxins, 15th edition. 1990 [ Links ]
11. Younis MHY, Kamal MM. TLC and HPLC assays of aflatoxins in Sudanese peanuts and peanut products. Kuwait J Sci Eng. 2003; 30:79-94. [ Links ]
12. Elamin NHH, Abdel- Rahim AM, Khalid AE. Aflatoxin contamination of groundnuts in Sudan. Mycopathol. 1988, 104: 25- 31. [ Links ]
13. Esameldin BMK, Salah Eldeen AA. Aflatoxins in Groundnut Paste in Khartoum State, Sudan, US Open Food Sci Technol J. 2014; 1 (3): 1- 8. [ Links ]
14. Shami EAB, Ahmed AAM. Survey and Determination of Aflatoxin Levels in Stored Peanut in Sudan, Jord J Bio Scie. 2011; 4(1):13-20. [ Links ]
15. Gilbert J, Shepherd MJ. (1985). A survey of aflatoxins in peanut butters, nuts and nut confectionery products by HPLC with fluorescence detection. Food Addit Contam. 1985; 2 (3): 171 -83. [ Links ]
Fecha de recepción: junio 2016. Fecha de aceptación: agosto 2016
Autor correspondiente:Salah Eldeen A. Ali.* University of Khartoum, Faculty of Science, Khartoum, Sudan
E-mail: salah_ncl@hotmail.com