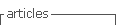Introduction
Endophthalmitis is a condition characterized by significant inflammation in the fluids and tissues inside the eye, that often leads to severe visual impairment. More frequently it is caused by exogenous pathogens following ocular surgeries, intraocular medication administration, penetrating eye injuries, or as an extension of a corneal infection. More rarely, endogenous pathogens can also cause endophthalmitis1.
The incidence of postoperative endophthalmitis following cataract surgery ranges from 0.056% to 1.3%., which rises to 30% after open-globe trauma. Notably, the primary source of postoperative endophthalmitis is the patient's bacterial flora found in the conjunctiva and eyelid2,3.
The onset of intraocular inflammation after microbial introduction can vary and depends on several factors, including the virulence of the microorganisms, the size of the initial infection, the patient's immune response, and the inflammatory reactions4.
Gram-positive staphylococci are responsible for over 90% of cases of postoperative infectious endophthalmitis, with Staphylococcus epidermidis accounting for approximately 70% of isolated microorganisms. Despite its relatively low virulence, once inside the eye, S. epidermidis can lead to severe and vision-threatening endophthalmitis2.
The destruction of intraocular structures, which can result in complete vision loss, occurs due to toxins and enzymes released by the microorganisms, as well as the response of the host's immune system5. There is existing evidence linking the ability of microorganisms like Staphylococcus aureus, Enterococcus faecalis, and Bacillus cereus to produce specific toxins with their virulence in endophthalmitis6-9. However, data on the effects of various strains of S. epidermidis in this context remain limited.
Bacterial products that play a significant role in biofilm formation are some of the most extensively studied virulence factors of Staphylococcus epidermidis10. These virulence determinants include the genes responsible for enhancing the virulence of Staphylococci, namely ICA (intercellular adhesin-operon - ICA ADBC). Within this group, icaA and icaD are responsible for the formation of biofilms, while the atlE gene codes for a protein crucial in the initial adherence of bacteria to surfaces11-13.
Previous research conducted by the authors involved assessing biofilm production through phenotypic methods in Coagulase-negative Staphylococci (CoNS) isolates14. This work revealed a high frequency of genes associated with virulence factors related to biofilm formation (ica and atlE), as well as the mecA gene, identified through a multiplex PCR assay, in coagulase-negative Staphylococcus isolates obtained from ocular samples of patients undergoing cataract surgery15,16.
Endophthalmitis animal models serve as valuable tools for unraveling the underlying mechanisms of this disease, depending on the infection source and the specific pathogen involved. These models enable researchers to explore various facets of the pathogenesis and pathophysiology of bacterial endophthalmitis, facilitating the development of anti-inflammatory treatment strategies and the evaluation of antibiotic pharmacokinetics and efficacy. While no single animal model can perfectly replicate the complexities of human bacterial endophthalmitis, researchers have successfully employed these models to gain insights into the infectious process, host responses, and potential therapeutic interventions17.
However, data from studies using animal models to investigate the relationship between microbial virulence and the severity of endophthalmitis remain limited. For instance, Mino de Kaspar et al. demonstrated in the rabbit model that the severity of inflammation correlated with the resistance pattern of the S. epidermidis strain responsible for causing endophthalmitis18. Resistant strains exhibited the ability to induce endophthalmitis in rabbits more rapidly and with greater severity than sensitive strains. Nonetheless, this study did not use molecular techniques to characterize the strains responsible for the infection.
To gain a deeper understanding of this phenomenon, our present study utilized the same animal model to assess whether mecA-positive (mecA+) and biofilm-producing S. epidermidis strains exhibit higher virulence compared to mecA-negative (mecA-) non-biofilm-producing strains. We evaluated clinical, ultrasound, and histopathological parameters to explore these differences comprehensively.
Methods
S. epidermidis strains
S. epidermidis strains used in this study exhibited varying levels of antimicrobial susceptibility and virulence gene profiles, as detailed in Table 1. The S. epidermidis strains were initially cultivated on blood agar plates at 37°C for a period of 24 hours. Subsequently, a single colony was selected from each strain and transferred into 10 ml of trypticase soy broth (TSB), where it underwent further incubation for an additional 24 hours at the same temperature, resulting in the creation of a stock solution. The bacterial organisms were then suspended in TSB to achieve an absorbance reading of 0.15 at 625 nm using a spectrophotometer, corresponding to a bacterial density of approximately 107 CFU/mL. To achieve the desired bacterial concentration for intravitreal injection, further dilution was carried out using sterile balanced salt solution. This final dilution was subsequently plated on trypticase soy agar to confirm the actual colony-forming units (CFU).
To ascertain the initial resistance pattern of each strain, antibiotic susceptibility testing was conducted using the Kirby-Bauer disc diffusion method. Mueller-Hinton agar (bioMérieux®, Stuttgart, Germany) was utilized for this purpose, following the guidelines established by the Clinical and Laboratory Standards Institute (CLSI).
The presence of genes encoding biofilm formation (ica and atlE) and gene encoding methicillin resistance (mecA) were detected by a multiplex PCR as described elsewhere1.
Animals
A group of 15 New Zealand white albino rabbits, each weighing between 2.0 and 2.5 kg, were selected for this study. These rabbits were individually housed in cages within a controlled environment, following a 12-hour light-dark cycle, and had unrestricted access to food and water. These accommodations were provided at the animal care facility of the Instituto de Investigaciones en Ciencias de la Salud at the National University of Asunción in Paraguay.
The well-being of the rabbits was carefully maintained, in compliance with the Guiding Principles for the Care and Use of Animals. The research team consisted of experts from various fields, including ophthalmologists, a veterinarian, a microbiologist, and a pathologist.
Inoculation
The fifteen rabbits were divided into two groups. In Group 1, seven rabbits were inoculated with highly virulent strains of S. epidermidis. Among them, four rabbits received a strain of S. epidermidis that demonstrated resistance to more than three antibiotics. This strain carried the mecA, ica, and atlE genes, and it was originally isolated from a patient who had undergone cataract surgery. The remaining three rabbits in Group 1 were inoculated with a multi-antibiotic resistant S. epidermidis strain, ATCC 35984 (Microbiologics, USA), known for its biofilm-forming capabilities. In Group 2, eight rabbits were inoculated with less virulent strains of S. epidermidis. Five of these rabbits received a strain isolated from a patient who had undergone cataract surgery.
This strain was sensitive to all tested antibiotics and did not carry the mecA, ica, and atlE genes. The remaining three rabbits in Group 2 were inoculated with a non-biofilm-forming S. epidermidis strain, ATCC 29122 (Microbiologics, USA).
All fifteen rabbits underwent intramuscular anesthesia through the administration of ketamine hydrochloride (35 mg/kg body weight) and lidocaine hydrochloride (5 mg/kg body weight). Before to bacterial inoculation, additional topical anesthesia was applied using 0.5% proparacaine hydrochloride eye drops. Under anesthesia, a paracentesis was created on the right eye, allowing for the aspiration of 0.1 ml of aqueous humor from the anterior chamber using a 30-gauge needle attached to a tuberculin syringe. Subsequently, 0.1 ml of the S. epidermidis suspension, containing 100 CFU, was introduced into the vitreous cavity of one eye in each rabbit via the pars plana, approximately 2 mm posterior to the limbus, using a 30-gauge needle on a tuberculin syringe. The other eye remained untreated and served as the control.
Slit-lamp biomicroscopy and indirect ophthalmoscopy
Following the inoculation procedure, slit-lamp biomicroscopy and indirect ophthalmoscopy were conducted at three-hour intervals until the emergence of endophthalmitis symptoms, and subsequently, at 24-hour intervals. Before to each examination, 1% tropicamide and 2.5% phenylephrine eye drops were applied to dilate the pupils. The severity of ocular inflammation was assessed based on anterior chamber reaction and fundus reflex, utilizing the Peyman et al. model as a reference (Table 2)19.
Ultrasound evaluation
After the application of topical anesthesia using 0.5% proparacaine hydrochloride eye drops into the conjunctival sac, a trans-palpebral ultrasound examination using a 10 MHz transducer was performed. To ensure accurate imaging, an abundant amount of methylcellulose gel was applied over the eyelid to prevent the formation of air bubbles between the transducer and the skin surface. Ocular and orbital examination was carried out systematically starting with a parasagittal plane through the center of the eye. From this initial plane, angling the transducer to the right and left, the sweep was made from the innermost part to the outer part of the organ studied. Then, the axial plane was explored, also through the center of the cornea and the vitreous chamber and angling the transducer from the top to the bottom, until the entire globe was observed.
Animal sacrifice
On the 15th day post-injection, the animals were humanely euthanized in a CO2 chamber. Subsequently, 0.1 ml of vitreous humor was aspirated using a 30-gauge needle attached to a tuberculin syringe, and the eyes were enucleated and fixed in a 10% formalin solution in phosphate-buffered saline for histopathological analysis.
Histopathological evaluation
For histopathological analysis, the eyes were embedded in paraffin, sectioned, and stained using standard protocols, including hematoxylin-eosin, periodic acid-Schiff, and Gram stain. Sections were carefully examined and assessed by an investigator who was unaware of the treatment group identities. A modified defined classification scheme was used to quantify the level of inflammation in various ocular structures, including the cornea, iris, vitreous base, ciliary body, and retina (Table 2). The assessment of retinal inflammation was performed at three distinct locations: the central retina, approximately 20° paracentral retina, and near the ora serrata. Evaluation of the retina was conducted on both the nasal and temporal sides to prevent any false readings due to localized swelling.
The histopathological evaluation involved categorizing the presence or absence of acute inflammation and classifying its degree into four categories: "no inflammation," "mild," "moderate," and "severe inflammation" for various anatomical structures such as the cornea, iris, ciliary body, choroid, vitreous, and retina. Scores were assigned based on the findings, as detailed in Table 2, with zero indicating uncompromised structures, one for mild involvement, two for moderate involvement, and three for severe involvement. The total possible score in the histopathological quantification was 12.
Results
Fifteen rabbits inoculated with S. epidermidis strains with two profiles of antibiotic susceptibility and virulence factors were studied. The clinical scores of the study eyes following intravitreal injection of these Staphylococcus epidermidis strains are presented in Table 3 and Table 4.
In the virulent strain-inoculated group (Group 1), all rabbits developed endophthalmitis, characterized by vitritis observed within 8 hours post-inoculation. This vitritis initially manifested as mild vitreous opacities, which progressed to severe by the end of the evaluation. Among the three rabbits inoculated with the biofilm-producing S. epidermidis ATCC 35984 strain, moderate vitreous opacity was observed 24 hours post-inoculation, with no significant changes thereafter. Clinical scores classified two rabbits as grade 3 and the remaining 4 as grade 2.
In contrast, the non-virulent group (Group 2) exhibited a milder response. One rabbit developed mild vitritis 24 hours post-inoculation, while two rabbits showed mild vitritis 8 hours post-inoculation, which escalated to moderate by the end of the evaluation. In one rabbit, moderate vitritis was evident 8 hours post-inoculation. Among the three rabbits inoculated with a strain of S. epidermidis ATCC 29122, a slight vitreous opacity was noted 24 hours post-inoculation, with no significant changes subsequently.
Clinical scores revealed higher severity in animals inoculated with the most virulent S. epidermidis strains compared to those inoculated with the less virulent strains (Table 4). At the 72-hour post-inoculation mark, the average score was significantly higher in the virulent group (p-value = 0.029) as depicted in Figure 1. Average score was significantly higher in the virulent group at 32 h (p value = 0,014) and 72 h post inoculation (Mann Whitney test p value = 0.019)

Table 3 Clinical grading of experimental eyes after intravitreal injection of staphylococcus epidermidis strains.
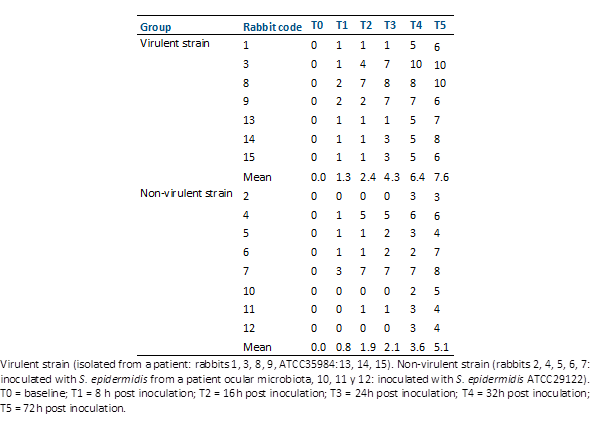
Table 4 Total clinical scores of experimental eyes after intravitreal injection of staphylococcus epidermidis strains.

Figure 1 Post-inoculation follow-up. Comparison of the total clinical scores of the eyes of rabbits inoculated with virulent and non-virulent strains of s. Epidermidis.
Ultrasound scans were conducted both before and after inoculation until clinical signs of endophthalmitis were confirmed. Initial scans showed no abnormalities in either group, with clear vitreous or no signs of inflammation. Subsequent scans revealed mild to moderate inflammatory vitreous condensations in two rabbits from the non-virulent strain-inoculated group (Group 2). In the virulent group (Group 1), one rabbit exhibited vitreous condensations with mild inflammatory characteristics, two rabbits displayed a moderate inflammatory appearance, and the remaining two rabbits had mild to moderate condensations (Figure 2). Initial ultrasound scans were normal in both groups, with clear vitreous and no inflammatory signs (A and C). In the follow-up scans, in the virulent group (Group 1) two rabbits had vitreous condensations with a mild to moderate inflammatory appearance (B), and one rabbit had vitreous condensations with an appearance of severe inflammation (D).
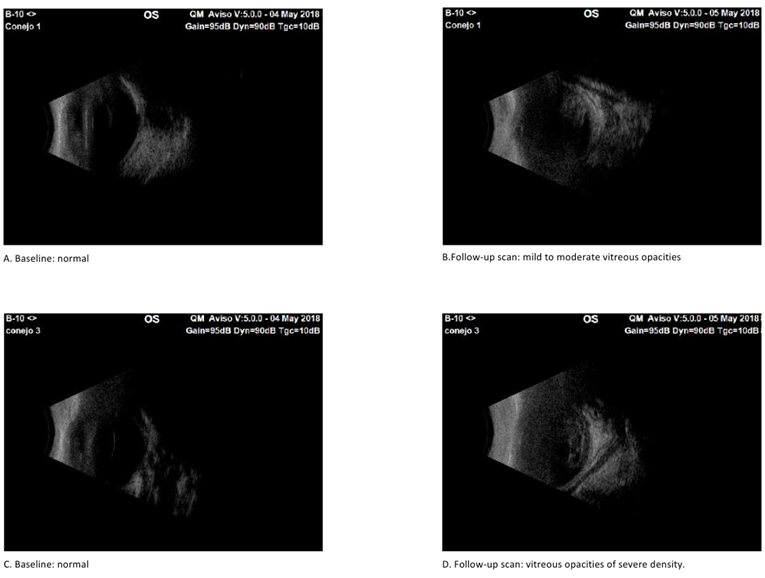
Figure 2 Sonographic results of experimental eyes after intravitreal injection of virulent and non-virulent strains of staphylococcus epidermidis.
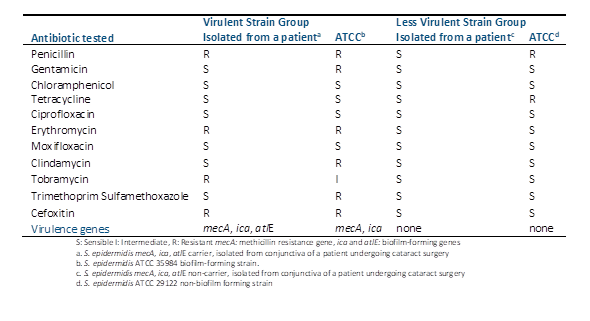
Histopathological examination unveiled varying degrees of inflammatory infiltration in the vitreous and retina, primarily consisting of polymorphonuclear leukocytes in both groups of rabbits (Figure 3). Rabbits inoculated with the most virulent S. epidermidis strain displayed, on average, higher histopathologic scores, indicating a greater presence of tissue inflammation compared to the group inoculated with the less virulent strain (Table 5).
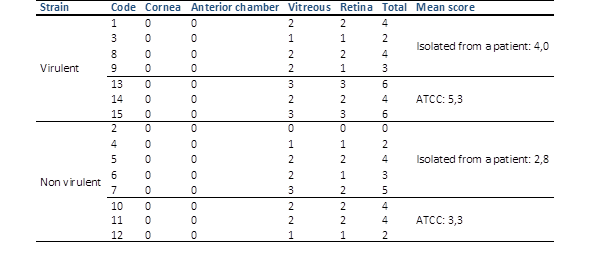
Table 5 Histopathologic grading of experimental eyes after intravitreal injection of the different groups of staphylococcus epidermidis.
Virulent strain (rabbits 1, 3, 8, 9: isolated from a patient; 13, 14, 15: ATCC). Non-virulent strain (rabbits 2, 4, 5, 6, 7: isolated from a patient; 10, 11, 12: ATCC). No significant difference (Kruskal Wallis test p value = 0.175)
In some rabbits inoculated with the more virulent S. epidermidis strains, abundant purulent exudative material composed mainly of polymorphonuclear leukocytes was observed, particularly in the posterior part of the vitreous. Additionally, retinal detachment accompanied by subretinal polymorphonuclear inflammatory infiltrate was noted. Conversely, histological sections of the eyes of one rabbit inoculated with the less virulent S. epidermidis strain revealed no purulent exudative changes or alterations in ocular structures (Figure 3).
Discussion
This study assesses the clinical outcomes of endophthalmitis induced by Staphylococcus epidermidis in rabbits, taking into consideration the antibiotic resistance and virulence-gene profiles of the infecting strain. Staphylococcus epidermidis strains are frequently resistant to multiple antibiotics, and their propensity to form biofilms poses a significant challenge for treatment. The capacity to form biofilms is recognized as the primary pathogenic factor20. Biofilm production and associated genes, particularly ica, have been proposed as markers for clinically significant Coagulase-negative Staphylococci strains. Some studies have indicated that S. epidermidis strains isolated from infected patients exhibit a higher propensity to produce biofilms compared to those isolated from normal flora21,22.
A clinical feature of infectious endophthalmitis is an inflammatory exudate which is apparent at an early stage. In the study, all animals developed some degree of infectious endophthalmitis. The clinical score was higher in those animals inoculated with the virulent strain (Group 1) and the time interval between inoculation and clinical signs of endophthalmitis was shorter. These results agree with the study carried out by Kaspar et al.18, where experimental endophthalmitis induced by fully susceptible coagulase-negative staphylococci resulted in a clinically distinct milder inflammatory response in the early stage compared to endophthalmitis resulting from partially resistant or multiresistant bacteria.
Another clinical feature of infectious endophthalmitis is inflammatory cell infiltration. Neutrophils play a key role in both bacterial clearance and inflammation in bacterial endophthalmitis. In the present study, the histopathological results showed a greater inflammatory reaction in the vitreous and retina, correlating with the clinical and ultrasound findings in rabbits from the virulent group, however the total histologic score was not significantly different between the two groups. The lack of significant difference in the histological results might be explained by the fact that the time of animal sacrifice was 15 days after inoculation as compared to 5 days carried out in the study by Kaspar et al18. One study in a murine model showed that while the bacterial burden gradually declines in the infected eye, neutrophil infiltration and retinal tissue damage continue resulting in significant vision loss23.
Meredith et al. 24 evaluated the development of endophthalmitis depending on the concentration of the inoculum and found that the initial inflammatory clinical signs were positively correlated with the size of the inoculum. The results of the present study show that S. epidermidis was able to cause endophthalmitis in rabbit eyes at a relatively low inoculum resulting in significant inflammation and retinal tissue damage after a short time-interval of less than 12 hours following bacterial inoculation. These findings highlight the need for appropriate preventive measures in the surgical setting and stress the importance of diligent povidone-iodine prophylaxis before cataract surgery. Once inside the eye, S. epidermidis can lead to severe and vision-threatening endophthalmitis2. Many studies report that the greatest reduction of the conjunctival bacterial load, which is used as a surrogate parameter for the risk of postoperative endophthalmitis, is achieved by povidone-iodine and not by topical antibiotics25-27. Furthermore, povidone-iodine has been shown to reduce the actual rate of postoperative endophthalmitis in a prospective trial by Speaker and Menikoff28. Other studies report a decline in the rate of infectious endophthalmitis after intraocular surgery and intravitreal injections following the introduction of standardized preoperative prophylaxis protocols including a flush-irrigation of the conjunctival sac with povidone-iodine29. Ophthalmologists must be vigilant of early signs of endophthalmitis.
In patients undergoing intraocular surgery, the identification of multiresistant and virulent genes CoNS carriers in the conjunctiva and eyelid by a suitable rapid molecular method should be developed and included in the routine laboratory testing. In those patients carrying these types of bacteria a more drastic protocol to ensure the elimination of these bacteria should be applied to reduce even more the incidence of endophthalmitis rate after intraocular surgeries, especially in the presence of intra surgery complications, such as rupture of the posterior capsule posterior - during cataract surgery.