Introduction
In recent years, non-psychoactive industrial hemp (Cannabis sativa L.) has experienced significant growth due to its usefulness in the textile, medicinal, and food industries (Rupasinghe et al., 2020). The cultivation of hemp in Paraguay has increased eight times, ranging from 600 ha in 2019 to 5000 ha in 2022 (Prohibition Partner, 2022). The reasons include its decriminalization and high demand in domestic and international markets Reuters (2023). The rapid expansion of organic crops has increased focus on pest management research. Nevertheless, limited information on the subject presents significant challenges to producers in effectively managing diseases caused by fungal and insect pests, which emerge as a result of high crop density and specific management practices implemented in the field (Sunoj Valiaparambil et al., 2023; Zheljazkov et al., 2023). Soil is considered one of the main sources of inoculum of phytopathogens (Johns et al., 2021). The phytopathogen Fusarium oxysporum has been found in soils of C. sativa crops in North America, Israel, and Italy, raising concerns among hemp growers worldwide due to the significant commercial losses. This pathogen can infect the plant from its seedling stage and spread through infected mother plants, generating varying degrees of aggressiveness depending on the haplotypes (Jerushalmi et al., 2022). Previous studies in C. sativa have revealed the presence of 12 haplotypes of Fusarium cannabis, of which five have been reported to cause significant economic losses due to plant diseases and mycotoxin residues (Gwinn et al., 2022). Hemp crops are also vulnerable to pest, such as the red spider mite (Tetranychus urticae), which can significantly reduce production and commercial product quality. To control this pest growers, use mites from the genera Neoseiulus californicus and Phytoseiulus persimilis as biological control agents. In addition, hemp farmers explored the potential of entomopathogenic fungi, whose effect as bioinsecticides is well-known in other crops (Jimenez Lara, 2022). In 2019 in Ecuador, Tetranychus urticae, caused 60- 80% losses in strawberry crops, which motivated the evaluation of the entomopathogenic activity of Bacillus subtilis in vitro (Mendoza-Léon et al., 2019). While the effects of fungi such as Beauveria bassiana and Metarhizium anisopliae as biocontrol agents are well-known, bacteria have been little studied. The results obtained were very encouraging and suggest that this technique could be used as a biocontrol alternative for other crops. (Pacheco Hernández et al., 2019). In Paraguay, during research looking to obtain biological control agents from the compost used to grow Cannabis seedlings, several genera of filamentous fungi were isolated and molecularly identified as Aspergillus sp., Penicillium sp., Cladosporium sp., Geomyces sp., Plectosphaerella sp., and Rasamsonia sp. These genera are common in crop soils, although Cladosporium sp. should be considered a potential threat in C. sativa as it is a pathogen in other crops (Álvarez Beauchamp, 2022). This research aimed to isolate and identify microscopic fungi associated with the soils and insects of organic non-psychoactive industrial hemp crops on a farm in the Eastern Region of Paraguay to characterize the diversity.
Materials and methods
Sampling site
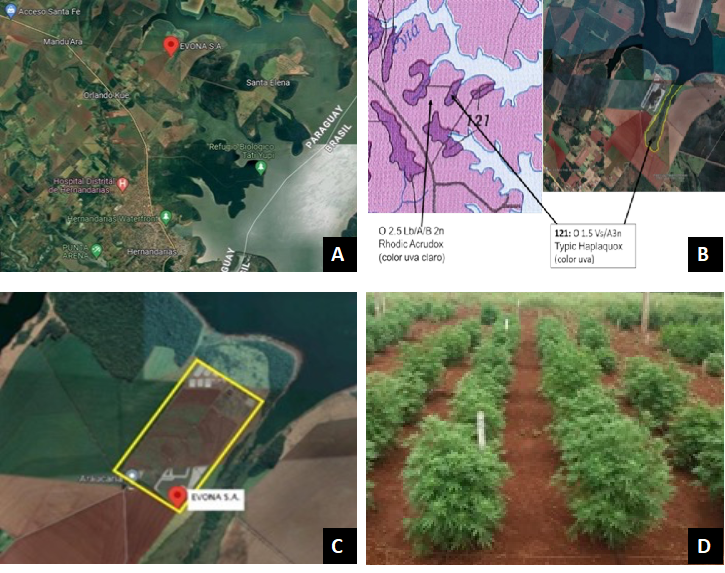
The sampling places were selected according to farmers that develop organic crops and previous investigations agreements. Samples were collected at nine georeferenced points selected randomly in the certified organic soils belonging to EVONA S.A. (Figure 1). The production fields were located in Hernandarias city, 7.32 km from route PY07 Km 28 (25°18'7"S 54°37'54"W). Georeferencing was performed using the Android operating system application GPS Fields Area Measure PRO.
Soil samples
According to data published by the Departamento de Planificación Ambiental del Ministerio de Agricultura (Paraguay), the soil type belongs to the Oxisol classification, soils derived from basalt that show an undulating profile in the form of hills followed by flat areas, the content of mica, apatites, feldspars and ferromagnesian minerals must be below 10% since they are weatherable.
Subsequently, soil samples weighing 200 grams each, 1800 grams for all, were extracted for pH determination and microbiological analysis. The soil samples came from places where hemp was grown between April and August 2022, in plots where hemp cultivation is anticipated in the future, and from residual substrate. The sample preparation followed the methodology proposed by Jerke et al., (2019). The soil samples were collected in magnetic-closure bags for transport to the Ciencia y Tecnología laboratory at the Universidad Nacional de Itapúa.
Solutions were prepared in sterile distilled water from nine soil samples to measure pH. Twenty grams of each sample was weighed and added to a beaker containing 50 ml of distilled water. The mixture was stirred with a glass rod for 2 minutes and left to settle for 15 minutes. The pH of the supernatant was measured using a QUIMIS Q400AS 240V-10W benchtop pH meter. To isolate fungi from the soil samples prepared for hemp cultivation, serial dilutions of 10-1, 10-2, and 10-3 were prepared in sterile distilled water. They were then seeded onto nutrient agar plates containing chloramphenicol antibiotic (150 mg/l). The plates were labeled and incubated at 25°C for 5 to 7 days until colony growth was observed (Gonzalez et al., 2013; Humber, 1997).
Insect samples
All dead insects found on the plants or laying on the soil were collected. The insect samples were transported sterile jars to the Ciencia y Tecnología laboratory (Universidad Nacional Itapúa). They were processed using the wet chamber technique and the direct puncture technique of the soft tissues, which were then cultured on potato dextrose agar (PDA) agar plates. The insects were identified in the field through morphological features.
Fungal colonies were observed both macroscopically and microscopically. For microscopic observations, a compound microscope (Zeiss Primo Stard Trinocular®) with an incorporated digital camera was used to capture images at magnifications of 400X and 1000X. The taxonomic guide of Dr. Humber (1997) was used for the microscopic identification of the fungal genus. The fungal colonies were observed in the Faculty of Science and Technology laboratory at UNI.
Results and Discussion
A total of nine samples of oxisol soils and 12 samples of insects were collected from the hemp plantations (Figure 2). The main insects genera found were Diabrotica, Empoasca, Naupactus and Autographa (personal communication with Ing. Alan Salinas, EVONA S.A.).
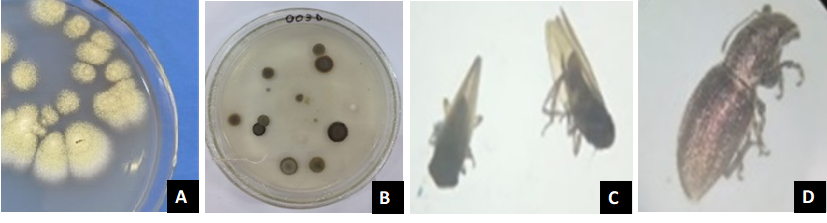
Figure 2. Examples of organisms-inhabiting in hemp plantations sampled in Paraguay. A and B fungal colonies isolated from soil samples. C and D insects collected from hem plantations.
Of the 42 fungal colonies growing in the Petri dishes with PDA from soil samples, 30 were confirmed through microscopic examination to represent one of the seven genera: a Rhizopus, Aspergillus, Absidia, Alternaria, Mucor, Penicillium, and Fusarium. In all the oxisol soils from the hemp plantation the genera Fusarium, Aspergillus, Penicillum and Cladosporium were found. The absence of entomopathogenic fungi of the species B. bassiana and M. anisopliae may be attributed to the acidic conditions of the soil and the high temperatures encountered at the various sampling sites. From the collected insects Cladosporium, Alternaria, Aspergillus, and Fusarium were isolated. In our study, all the samples were collected during the continuous dry season prevailing in our region, with a remarkably absence of regular rainfalls, which may alter the occurrence of many groups of microorganisms from samples of soil. It has been suggested that the presence of B. bassiana to be very low during periods of scarce rainfall and intense drought, increasing during rainy months with higher humidity and temperatures of 20-30°C, conditions favorable for spore dispersal and germination. However, the genus M. anisopliae was found to invade tissues of dead insects when temperatures reached 40°C. These data highlight the importance of climatic conditions in the development of some pathogens and emphasize the need to consider them in future studies of pathogenicity related to hemp cultivation (García-García et al., 2011). Another important aspect to consider is that the pH in the analyzed soils was rather acid, varying between 5.2 and 5.71. The field frequency of entomopathogenic fungi was low, which could be related to crop management, environmental conditions, availability of susceptible hosts, pathogen virulence, and host crop characteristics, among others (Borges, 2007; Vänninen, 1996). Climatic factors such as moderate temperatures, abundant rainfalls, and high humidity are correlated with the occurrence of entomopathogenic fungi (Clifton et al., 2015; Jaronski, 2010; Yaginuma, 2007). Similarly, Silva Guerra et al., (2009) reported the effect of moderated temperatures and high percentajes of humidity favours the persistence of conidia in soil, where M. anisopliae was found to be more resistant than M. acridum . The presence of Penicillum, Alternaria, Aspergillus, and Fusarium genera in soil and pest insect tissues would merit further study of their pathogenicity under controlled laboratory conditions to deepen knowledge of their potential use as biocontrol agents of hemp crop pests.
Conclusions
This work has made it possible to know the fungal diversity present in soils and pest insects associated with organic crops of industrial hemp (Cannabis sativa, L.) for the first time in Hernandarias, Alto Paraná, Paraguay. All of the isolated and identified fungi of the genera Alternaria, Rhizopus, Absidia, Mucor, Penicillium, Aspergillus, Fusarium, and Cladosporium have been stored in the InBioMis culture collection, Misiones Argentina, and the Faculty of Science and Technology of the National University of Itapúa for future molecular identification assays.
The present work allowed us to establish links between the National University of Itapúa, the Institute of Biotechnology of Misiones, Argentina, and the company Evona S.A. so that we can continue researching and interacting in the future for the benefit of organic production of industrial hemp.











 uBio
uBio