1. INTRODUCTION
Cancer is a target of research for the development or discovery of new forms of treatments1. Many drugs used in cancer chemotherapy, like doxorubicin (DXR), a widely used drug for different cancer types2, have low specificity that results in undesirable effects. Studies show that DXR cause toxicity in both primordial follicles and growing ovarian follicles, triggering follicular and oocyte apoptosis3, and eventually affect human fertility. Therefore, plants are excellent sources of raw material when searching for new anticancer drugs4.
Auxemma. oncocalyx (A. oncocalyx) is a common tree found in the state of Ceará in Northeast Brazil 5. It has been widely used in folk medicine as an adjunctive treatment of injuries such as wounds and cuts 5,6. Some studies have suggested that this plant has biological activities such as analgesic, antioxidant, antitumor and anti-inflammatory effects6-9. Oncocalyxone A (onco A) is A. oncocalyx’s active compound. Onco A has high antioxidant activity6 and an anti-proliferative effect on tumor cell cultures10. Studies have suggested onco A as a possible anticancer compound since it presents antitumor and cytotoxic activity in human leukemia cells, and other cell cancer lines, without causing genotoxicity11 las most anticancer drugs.
Little is known about reproductive toxicity of A. oncocalyx and onco A in mammals. In recent pioneer studies conducted by our group with caprine preantral follicles cultured in vitro enclosed in ovarian cortical tissue, A. oncocalyx and onco A affected in vitro caprine early folliculogenesis in a concentration-dependent manner12. However, no toxic effect of A. oncocalyx and onco A was observed on in vitro development of late caprine isolated secondary follicles. In contrast, these drugs affected the cumulus-oocyte complexes (COCs) viability after in vitro maturation but not the metaphase II rates (Leiva-Revilla et al., 2016 b - under review). In both studies, DXR was used as positive (toxic) control and presented a more toxic effect than A. oncocalyx and onco A. These results suggest that A. oncocalyx and onco A despite of having anticancer effects they are, apparently, less harmful to reproductive parameters than commercial drugs, such as DXR.
Normal embryonic development is preceded by a sequence of coordinate events during maturation and fertilization. The mechanism of oocyte maturation encompasses interactions between the oocyte and its surrounding cumulus cells, which synchronizes meiosis with structural and molecular changes in the ooplasm, enabling the oocyte to support proper fertilization and subsequent embryo development13. Consequently, it is of high significance to study the toxic effect of new drugs over in vitro maturation, fertilization and embryo development.
To the best our knowledge, there is no information about the influence of A. oncocalyx and onco A on the in vitro embryo development in mammals, including pigs. Compared to the other species, the porcine seems to be a suitable animal model for humans, due to the ovarian similarities14. In addition, the advantage of using pig ovaries is that the ovaries are from animals at similar age, breed and controlled nutrition. Thus, the porcine specie has been quite used as a model for human oocytes in toxicity tests15.
A. oncocalyx and onco A are being studied as possible anticancer agents, most anticancer treatments are known to have harmful effects on reproduction. The aim of this study is to analyze the effects of A. oncocalyx and onco A on some reproductive parameters and to compare whether it is the same, less or more harmful than a known anticancer (DXR). Therefore, we evaluate the effect of A. oncocalyx, onco A and DXR exposure during in vitro maturation of oocytes (Experiment 1) or in vitro embryo culture (Experiment 2) on the oocyte developmental competence, investigating the following end points: oocyte viability, maturation rates and efficiency, in vitro fertilization parameters and percentage of cleaved embryos and blastocyst formation.
2. METHODOLOGY
2.1. Culture media
All chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise indicated. The medium used for the collection of cumulus-oocyte complexes (COCs) and for washing was Dulbecco’s phosphate-buffered saline (DPBS) medium composed of 136.89 mM NaCl, 2.68 mM KCl, 8.1 mM Na2HPO4 and 1.46 mM CaCl2·2H2O supplemented with 4 mg/mL bovine serum albumin (BSA), 0.34 mM sodium pyruvate, 5.4 mM D-glucose and 70 µg/mL kanamycin (mDPBS). The oocyte maturation medium was modified-TCM 199supplemented with 150 µM cisteamine and 10 ng/m Lepidermal growth factor(TCM-199+)15. The basic medium used for fertilization was essentially the same as that used by Abeydeera and Day16. This medium, designated as a modified Tris-buffered medium, consisted of 113.1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2·2H2O, 20 mM Tris (crystallized free base), 11 mM glucose and 5 mM sodium pyruvate supplemented with 2 mM caffeine and 0.2% BSA. The embryo culture medium was a sequential medium based on North Carolina State University (NCSU)-23 Medium supplemented with 0.4% BSA15.
2.2. Isolation of onco A from A. oncocalyx
The obtaining of the A. oncocalyx and onco A has previously been described by Pessoa et al. (11). Briefly, A. oncocalyx was collected and identified by Dr. Maria Iracema B. Loiola of the Department of Biology of Federal University of Ceará. Onco A (C17H18O5) was extracted from woody parts of A. oncocalyx (Boraginaceae) by phytochemical extraction methods using organic solvents, and was isolated and purified by crystallization and recrystallization. It is noteworthy that the fraction of A. oncocalyx contains 80 % of onco A (9), therefore the concentration of A. oncocalyx was in equal proportion of onco A. A. oncocalyx and onco A were diluted with DMSO as a vehicle. The concentrations of A. oncocalyx and onco A were chosen based on previous studies performed in our laboratory17.
2.3. Experimental design
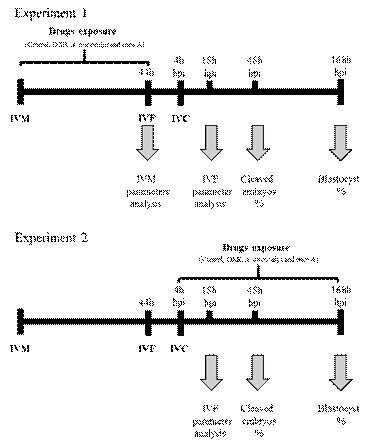
Experiment 1: Effect of A. oncocalyx and onco A on IVM of porcine COCs and subsequent embryo development.
In this experiment, we evaluated the effect of DXR, A. oncocalyx and onco A on in vitro maturation (IVM) of porcine COCs and subsequent embryo development (Figure 1). Immediately after oocyte recovery, COCs in vitro maturated in four treatments: I) TCM-199+ alone (control), or supplemented with II) 0.3 (g/mL DXR; III) 1.2 (g/mL A. oncocalyx or IV) 1 (g/mL onco A. After IVM, oocyte chromatin configuration and viability were assessed. Moreover, in vitro fertilization (IVF) was performed and 18 hours post insemination (hpi) we assess fertilization parameters, and finally, after 7 (168 hpi) days we evaluated embryo development.
Experiment 2: Effect of A. oncocalyx and onco A on the in vitro culture of porcine embryos.
COCs were submitted to IVM and IVF as described for experiment 1 (control group), then, zygotes were randomly allocated into four treatments for in vitro embryo culture (IVC): I) NCSU-23 alone (control) or supplemented with II) 0.3 (g/mL DXR; III) 1.2 (g/mL A. oncocalyx or IV) 1 (g/mL onco A. The presumptive zygotes were cultured for 18 hpi to assess fertilization parameters, and for 7 days (168 hpi) to evaluate embryo development (Figure 1).
2.4. Oocyte collection and IVM
Ovaries were obtained from prepuberal gilts at a local slaughterhouse and transported in 0.9% NaCl containing 70 µg/mL kanamycin, at 33°C within 1 h. In the laboratory, COCs were aspirated from medium-sized follicles (3 to 6 mm in diameter) using an 18-gauge needle connected to a 10-mL disposable syringe. Oocytes with a compact cumulus mass and a dark, evenly granulated cytoplasm were washed three times in maturation medium, and 50-60 oocytes were transferred into each well of a 4-well multidish (Nunc, Roskilde, Denmark) containing 500-µL of maturation medium supplemented with 10 IU/mL pregnant mare's serum gonadotropin and 10 IU/mL human chorionic gonadotropin for 20-22 h. The oocytes were then incubated for another 20-22 h in maturation medium without hormones. Oocyte maturation was carried out under mineral oil at 39ºC in a humidified atmosphere of 5% CO2 in air. After maturation, COCs were mechanically denuded 15).
2.5. In vitro fertilization and embryo culture
Groups of 30 denuded oocytes were placed in 50-µL drops of fertilization medium in a 35x10-mm Petri dish under mineral oil and held at 38.5ºC in an atmosphere of 5% CO2 in air. Pool of freshly ejaculated semen, diluted in extender from three boars from a local breeding station, was washed three times by centrifugation at 1900 x g for 3 min in mDPBS. The resulting pellet was re-suspended in fertilization medium, and after the appropriate dilution, 50 µL was added to a 50µL drop of fertilization medium containing the oocytes (2000:1 spermatozoa:oocyte ratio). The gametes were co-incubated at 38.5ºC in a humidified atmosphere of 5% CO2 in air for approximately 4 h.
Presumptive zygotes were removed from the fertilization medium and washed three times in pre-equilibrated embryo culture medium. The zygotes were then transferred to a 4-well multidish (30 zygotes per well), with each well containing 500 µL of the same medium under mineral oil, and were cultured at 38.5ºC in a humidified atmosphere of 5% CO2 in air. Presumptive zygotes were cultured for the first 2 days (Day 0 = day of fertilization) in glucose-free NCSU-23 supplemented with 0.33 mM pyruvate and 4.5 mM lactate and then in fresh NCSU-23 medium containing 5.5 mM glucose until day 7.
2.6. Assessment of oocyte chromatin configuration, viability, sperm penetration and embryo development
To evaluate maturation and fertilization parameters, denuded oocytes and presumptive zygotes were washed in PBS-BSA, and incubated in 500 µL droplets containing 4 µM calcein-AM, 2 µM ethidium homodimer-1 (Molecular Probes, Invitrogen, Karlsruhe, Germany), 0.5% of glutaraldehyde and 10 µM Hoechst 33342 for 15 min.
The maturation rate was assessed at 44h of IVM. The chromatin configuration patterns were the following: abnormal chromatin configuration, germinal vesicle (GV) or meiotic resumption. Meiotic resumption was defined when the nucleus was in germinal vesicle break down (GVBD), metaphase I (MI) or in metaphase II (MII) stages. Maturation efficiency was calculated by MII/total oocytes cultured. Thereafter, oocytes were also examined under a fluorescence microscope (Nikon, Eclipse 80i, Tokyo, Japan) for evaluation of live/dead fluorescent staining. The emitted fluorescent signals of calcein-AM and ethidium homodimer-1 were collected at 488 and 568 nm, respectively. Oocytes were considered viable when the cytoplasm was stained positively with calcein-AM (green) and chromatin was not labelled with ethidium homodimer-1 (red) and they showed a normal chromatin configuration.
Fertilization parameters were evaluated at 18 hpi. The oocytes were considered penetrated when they contained one or more swollen sperm heads and/or male pronuclei, with their corresponding sperm tails, and two polar bodies. The fertilization parameters evaluated were penetration rate (number of oocytes penetrated/total matured), monospermy (number of oocytes containing only one male pronucleus/total penetrated), number of spermatozoa/oocyte (mean number of spermatozoa in penetrated oocytes), and efficiency of fertilization (number of monospermic oocytes/total inseminated).
At 2 and 7 days after IVF, the cleavage rate (number of oocytes divided to 2-4 cells/total) and blastocyst formation rate (number of blastocyst/total cleaved), respectively, were evaluated under a stereomicroscope. An embryo that had cleaved to the two-cell stage or beyond was counted as cleaved, and an embryo with a clear blastocele was defined as a blastocyst
2.7. Statistical analyses
Statistical analysis was carried out with Sigma Plot 11.0 (Systat Software Inc., USA). Variables presented as percentage were analysed by chi-square or Fisher's exact tests. Comparison of means among treatments (n° spermatozoa/oocyte) was evaluated by Kruskal-Wallis test. Data are presented as mean (± SEM) or percentage and P < 0.05 indicates significant difference.
3. RESULTS
3.1. Experiment 1. Effect of A. oncocalyx and onco A on IVM of porcine COCs and subsequent embryo development
The rates of oocyte viability and maturation after exposure to DXR, A. oncocalyx and onco A are shown (Table 1). After IVM, the DXR, A. oncocalyx and onco A treatments showed a significant lower percentage of oocyte viability and maturation than control. Except for oocyte viability, A. oncocalyx and onco A showed similar results for the endpoints above being both higher (P < 0.05) than DXR. However, when only viable oocytes were considered to calculate the maturation rate no differences were observed among treated groups (DXR, A. oncocalyx and onco A). To determine the meiotic resumption and MI rates only viable oocytes were used. The percentage of meiotic resumption in the onco A treatment and rate of MI in the A. oncocalyx and onco A treatments were similar to DXR but higher (P < 0.05) than control.
The exposure of COCs to DXR, A. oncocalyx and onco A during IVM reduced significantly the IVF efficiency when compared to the control treatment (Table 2). However, when only matured oocytes were taken into account the penetration and monospermy rates as well as the number of spermatozoa/oocyte were similar (P > 0.05) among the treatments.
When the COCs were exposed to the three tested drugs during IVM (Figure 2), DXR, A. oncocalyx and onco A treatments showed lower (P < 0.05) cleaved rates compared to control. This endpoint was higher in the onco A treatment than DXR and A. oncocalyx treatments. With regard to blastocyst rate, lower (P < 0.05) values were observed in the DXR treatment.

Figure 2: Experiment 1. Percentage of cleaved (A) and blastocyst/cleaved (B) after previous exposure (only during in vitro maturation) to DXR, A. oncocalyx or onco A. a,b,c Distinct letters represent significant differences among treatments (P < 0.05).
Table 1: Rates of viable oocytes, germinal vesicle (GV), meiotic resumption and metaphase II (MII) rates, after in vitro maturation of porcine oocytes in control medium alone or supplemented with DXR, A. oncocalyx or onco A (experiment 1).
| Treatments | Total | Viable oocytes | Oocyte chromatin configuration | Maturation Efficiency | ||||
| GV | Meiotic resumption* | GVBD | MI | MII | MII / total | |||
| (n) | % | % | % | % | % | % | % | |
| Control | 157 | 81.53 (128/157) A | 7.81 (10/128) A | 92.19 (118/128) B | 2.34 (3/128) | 26.56 (34/128) B | 63.28 (81/128) A | 51.59 (81/157) A |
| DXR | 187 | 12.83 (24/187) D | 8.33 (2/24) A | 91.67 (22/24) AB | - | 45.83 (11/24) AB | 45.83 (11/24) AB | 5.88 (11/187) C |
| A. oncocalyx | 181 | 48.62 (88/181) C | 7.95 (7/88) A | 92.05 (81/88) B | - | 59.09 (52/88) A | 32.95 (29/88) B | 16.02 (29/181) B |
| Onco A | 163 | 59.51 (97/163) B | 1.03 (1/97) A | 98.97 (96/97) A | - | 64.95 (63/97) A | 34.02 (33/97) B | 20.25 (33/163) B |
A,B,C,D Distinct capital letters represent significant differences among treatments (P < 0.05). n Total number of analyzed oocytes per treatment.
* Includes GVBD, MI and MII oocytes.
3.2. Experiment 2. Effect of A. oncocalyx and onco A on the in vitro culture of porcine embryos
In the experiment 2 (Table 3), there was no difference (P > 0.05) between control group and other treatments regarding to viability and maturation rates. However, DXR and onco A showed a lower percentage (P < 0.05) of viability than A. oncocalyx. All the tested treatments reduced (P < 0.05) the penetration rate compared to control treatment. Onco A reduced significantly the monospermy and IVF efficiency, and increased (P < 0.05) the number of spermatozoa/oocyte. The IVF efficiency was also reduced (P < 0.05) in the DXR group.
The exposure of presumptive zygotes to onco A did not change either the cleavage or blastocyst rates comparing control (Figure 3). The addition of DXR and A. oncocalyx only reduced (P < 0.05) the cleavage rate.

Figure 3: Experiment 2. Percentage of cleaved (A) and blastocyst / cleaved (B) after exposure (only during in vitro embryo culture) to DXR, A. oncocalyx or onco A. a,b Distinct letters represent significant differences among treatments (P < 0.05).
Table 2: Rates of viable oocytes, matured, penetrated, monospermy and efficiency rates and number of spermatozoa per oocyte after previous exposure (only during in vitro maturation) to DXR, A. oncocalyx or onco A(experiment 1).
| Endpoints * | |||||||
| Treatments | Total | Viability | Matured / viable | Penetrated / matured | Monospermy / penetrated | IVF Efficiency (2pn/total) | # SPZ / oocyte |
| (n) | % | % | % | % | % | % | |
| Control | 251 | 78.49 (197/251) A | 96.95 (191/197) A | 67.02 (128/191) A | 57.03 (73/128) A | 29.08 (73/251) A | 1.94+ 0.14 A |
| DXR | 170 | 38.82 (66/170) C | 93.93 (62/66) A | 77.42 (48/62) A | 52.08 (25/48) A | 14.71 (25/170) B | 1.72+ 0.17 A |
| A. oncocalyx | 291 | 68.73 (200/291) B | 77.50 (155/200) B | 76.77 (119/155) A | 45.38 (54/119) A | 18.56 (54/291) B | 2.15+ 0.13 A |
| Onco A | 252 | 66.67 (168/252) B | 83.92 (141/168) B | 69.50 (98/141) A | 51.02 (50/98) A | 19.84 (50/252) B | 2.15 + 0.2 A |
A,B,C Distinct capital letters represent significant differences among treatments (P < 0.05). n Total number of analyzed oocytes per treatment. * All the endpoints were evaluated 18 hpi.
Table 3: Rates of viable oocytes, matured, penetrated, monospermy and efficiency rates and number of spermatozoa per oocyte after 18hpi exposure (only during in vitro embryo culture) to DXR, A. oncocalyx or onco A (experiment 2).
| Endpoints * | |||||||
| Treatments | Total | Viability | Matured / viable | Penetrated / matured | Monospermy / penetrated | Efficiency (2pn/total) | # SPZ / oocyte |
| (n) | % | % | % | _% | % | % | |
| Control | 71 | 71.83 (51/71) AB | 96.07 (49/51) A | 85.71(42/49) A | 54.76 (23/42) A | 32.39 (23/71) A | 1.43 + 0.1 B |
| DXR | 87 | 59.7 (52/87) B | 98.07 (51/52) A | 64.7 (33/51) B | 45.45 (15/33) AB | 17.24 (15/87) BC | 1.86+ 0.16 AB |
| A. oncocalyx | 88 | 75 (66/88) A | 93.93 (62/66) A | 69.35 (43/62) B | 51.16 (22/43) AB | 25 (22/88) AC | 1.72+ 0.14 B |
| Onco A | 73 | 58.9 (43/73) B | 97.67 (42/43) A | 59.52 (25/42) B | 28 (7/25) B | 9.59 (7/73) B | 2.51 + 0.28 A |
A,B,C Distinct capital letters represent significant differences among treatments (P < 0.05). n Total number of analyzed oocytes per treatment. * All the endpoints were evaluated 18 hpi.
4. DISCUSSION AND CONCLUSION
To our knowledge, the present study demonstrated for the first time the effect of A. oncocalyx and its isolated compound, onco A, on the in vitro maturation of porcine oocytes and subsequent in vitro embryo development.
When the three tested drugs exposure occurred only during IVM (Experiment 1), DXR, A. oncocalyx and onco A had a detrimental effect on the oocyte viability, being DXR the most toxic with only 12.83 % viable oocytes after 44h of exposure. It is known that DXR acts on several levels by different molecular mechanisms including an interaction with iron, upsetting calcium homeostasis, altering the activity of intracellular or intra-mitochondrial oxidant enzymes, and binding to topoisomerases (TOPOs) promoting their dysfunction leading to DNA damage and apoptosis18. Specifically, DXR acts by inhibiting Topoisomerase II (Topo II). In the female, Topo II is required for chromosome separation during oocyte meiotic maturation, but is dispensable for resumption of meiosis19. On the other hand, A. oncocalyx and onco A also disrupt oocyte viability and caused damage in the chromatin configuration. Sbardelotto20 showed that, in human promyelocytic leukemia line (HL-60) cells, onco A activates first the intrinsic apoptotic pathway by caspase 8, and then the extrinsic pathway by caspase 3 and 7. Contrary to DXR, onco A does not affect the TOPOs. However, in HL-60 cells, onco A cleaved poly (ADP-ribose) polymerase (PARP)20. PARP binds and repair DNA-strand breaks generated by genotoxic agents. Likewise, PARP is implicated in the regulation of a wide range of important cellular processes including transcriptional regulation, chromatin modification, cellular homeostasis, and cell proliferation and death21. Therefore, cleaved PARP results in an oocyte proapoptotic protein22. A study conducted in porcine showed that the cleavage of PARP1 was strongly implicated in follicular development and atresia of fetal, neonatal, and adult porcine ovaries 21. In addition, PARP-1 synthesize poly(ADP-ribose) (PAR), which is required for assembly and function of the bipolar spindle23. PARP-1 also mediates the regulation of centrosome duplication and chromosomal stability. The inhibition of PARP-1 is associated with mislocalization of centromeric and centrosomal proteins, defective chromatin modifications and genomic instability characterized by loss of mitotic checkpoint integrity24.
In the present study, the IVM efficiency was compromised in all treatments compared to control treatment. Interestingly, onco A increased the meiotic resumption rates. However, these oocytes were arrested at the MI stage. Anticancer drugs can cause double-strand breaks (DSBs). These DSBs do not arrest mouse oocytes in the G2/prophase but, instead, allow them to progress to the MI stage25. The exposure of oocytes to genotoxic agents, such as PARP inhibitors, causes failures in the spindle assembly checkpoint, leading to an oocyte meiotic arrest at MI25. Another study showed that damage in the microtubules, main structural elements of the spindle, result in oocytes arrest at the MI stage during in vitro maturation in mouse26. Our results suggest that onco A might be affecting the oocyte chromatin configuration, through PARP inhibition, leading to failures in the spindle assembly checkpoint and therefore causing this meiotic arrest at the MI stage.
In the present study, the exposure of COCs to the tested drugs only during IVM negatively affected the IVF efficiency. Even tough DXR, A. oncocalyx and onco A reduced embryo cleavage rate, only DXR showed a more toxic effect on blastocyst development. DXR elicits apoptosis by various mechanisms in a variety of cells. DXR is capable to accumulate in both nucleus and mitochondria and induce chromosomal obliteration by inhibiting Topo-II. In oocytes, it can interfere with mitochondrial function and start the intrinsic pathway of apoptosis via the mitochondria by reducing the mitochondrial membrane potential and releasing cytochrome C27. Impaired mitochondrial function lead to improper fertilization and a reduction of embryo development28.
When DXR, A. oncocalyx and onco A were added only during the in vitro embryo culture (Experiment 2), all drugs negatively affected the penetration rate evaluated after 18 hpi. However, DXR and onco A showed a detrimental effect on IVF efficiency. Nonetheless, only onco A augmented the percentage of spermatozoa per oocyte. Ferreira et al.29 showed that onco A was able to inhibit platelet aggregation by increasing the cyclic guanosine monophosphate (cGMP) levels in platelets by a synergistic mechanism, combining increased production and reduced degradation of cGMP 29. In porcine, cGMP activates cGMP-dependent protein kinase (PKG). This pathway plays an essential role in acrosome reaction, which enables the spermatozoa to penetrate the zona pellucida, and therefore, to fuse with the oocyte plasma membrane30. Zhang31 observed that when a cGMP analog, atrial natriuretic peptide (ANP), was added during IVF of frozen-thawed giant panda sperm with porcine salt-stored oocytes, it resulted in a higher proportion of oocytes with spermatozoa in the zona pellucida and perivitelline space, and a higher average number of spermatozoa/oocyte.
In experiment 2, DXR, A. oncocalyx and onco A negatively affected the cleavage rate. Wang et al.32 showed that DXR blocked pre-implantation development in early mouse embryos by altering apoptosis-related gene expression, Bcl2l1 and Casp3, and inactivating DNA repair by PARP. In the same study, the authors found out that DXR arrested zygotes at the 1-cell stage by disruption of DNA and of the cytoskeleton. In addition, it is known that during blastocyst formation, the inhibition PARP suppresses selective autophagic degradation of ubiquitinated proteins, which contributes to apoptosis 33. Thus, the interaction between PARP and autophagy influences the quality of in vitro produced embryos in porcine34. Due to the fact that onco A affects PARP in HL-60 cells (20), and that A. oncocalyx contains 80% of onco A in its composition9, both drugs could be affecting embryonic competition by this pathway. Moreover, a study evaluating different concentrations (1 to 100 µg/mL) of a quinone fraction of A. oncocalyx in sea urchin eggs reported that the cleavage of eggs was inhibited in a concentration-dependent manner35. Despite the fact that DXR, A. oncocalyx and onco A reduced porcine embryo cleavage rate, they did not affect blastocyst development, showing that porcine blastocyst tend to be more resistant to toxic agents36.
As expected DXR caused deleterious effects on the evaluated reproductive parameters; in brief, the addition of DXR during IVM or IVC negatively affected the IVF efficiency and cleavage rate. Even though both. A. oncocalyx and onco A, impair in vitro porcine oocyte developmental competence, they were less detrimental than DXR in oocyte viability and blastocyst formation.
In conclusion, although A. oncocalyx and onco A showed toxic behavior, they are less harmful than DXR in the reproductive parameters studied. Therefore, more studies are required to determine if it really would be a less toxic treatment alternative than conventional anticancer in terms of reproductive harm.











 uBio
uBio